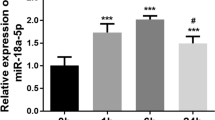
Abstract
Isoflurane is one of the most frequently used volatile anesthetics in clinical practice for inhalational anesthesia. It is widely studied that isoflurane mediates cardioprotection during multiple pathological processes. However, the precise mechanisms have not been fully elucidated. Neonatal cardiomyocytes were isolated and cultured, followed by treatments with isoflurane at 0, 50, 100 or 200 µM. Rat cardiomyoblast cell line, H9c2, was treated with H2O2. Expression of miR-23 was measured by qRT-PCR. The cell survival rate of H9c2 in response to H2O2 treatments was evaluated by MTT assay. The ROS and GSH/GSSG levels were measured using Superoxide Detection Kit and GSH/GSSG Ratio Detection Assay Kit. In this study, we report an isoflurane-miR-23-antioxidant axis in cardiomyocyte. We observed that miR-23 was suppressed by isoflurane treatments at 50, 100 or 200 µM. Moreover, cardiomyocyte with isoflurane exposure was insensitive to H2O2 treatment in vitro. Inhibition of miR-23 protected cardiomyocyte against oxidative stress induced by H2O2 treatments at 30, 60, 90 or 120 µM. In addition, overexpression of miR-23 induced ROS generation over twofolds and rendered cardiomyocyte sensitive to H2O2 treatments. We demonstrate that miR-23 inhibited intracellular GSH, an antioxidant against oxidative stress. Our results reveal that with isoflurane exposure, overexpression of miR-23 rendered cardiomyocyte sensitive to H2O2 treatments at 20, 30, 40, 50 µM. Pretreatments with GSH in miR-23 overexpressing cells rescued the cell death under oxidative stress. In summary, our results illustrate that the isoflurane-mediated protection of cardiomyocytes under oxidative stress is through inhibition of miR-23. This study provides an aspect for the miRNAs-modulated cardiomyocyte sensitivity to oxidative stress, contributing to the development of therapeutic agents.





Similar content being viewed by others
Abbreviations
- Isoflurane:
-
2-Chloro-2-(difluoromethoxy)-1,1,1-trifluoro-ethane
- I/R:
-
Ischemia–reperfusion
- ROS:
-
Reactive oxygen species
References
Van Allen, N. R., Krafft, P. R., Leitzke, A. S., Applegate, R. N., Tang, J., & Zhang, J. H. (2012). The role of volatile anesthetics in cardioprotection: A systematic review. Medical Gas Research, 2(1), 22.
Fan, W., Liu, Q., Zhu, X., Wu, Z., Li, D., Huang, F., et al. (2016). Regulatory effects of anesthetics on nitric oxide. Life Sciences, 151, 76–85.
Uhlig, C., Bluth, T., Schwarz, K., Deckert, S., Heinrich, L., De Hert, S., et al. (2016). Effects of volatile anesthetics on mortality and postoperative pulmonary and other complications in patients undergoing surgery: A systematic review and meta-analysis. Anesthesiology, 124(6), 1230–1245.
Jia, P., Teng, J., Zou, J., Fang, Y., Zhang, X., Bosnjak, Z. J., et al. (2013). miR-21 contributes to xenon-conferred amelioration of renal ischemia-reperfusion injury in mice. Anesthesiology, 119(3), 621–630.
Lang, X. E., Wang, X., Zhang, K. R., Lv, J. Y., Jin, J. H., & Li, Q. S. (2013). Isoflurane preconditioning confers cardioprotection by activation of ALDH2. PLoS ONE, 8(2), e52469.
Raphael, J., Rivo, J., & Gozal, Y. (2005). Isoflurane-induced myocardial preconditioning is dependent on phosphatidylinositol-3-kinase/Akt signalling. British Journal of Anaesthesia, 95(6), 756–763.
Chimenti, C., Scopelliti, F., Vulpis, E., Tafani, M., Villanova, L., Verardo, R., et al. (2015). Increased oxidative stress contributes to cardiomyocyte dysfunction and death in patients with Fabry disease cardiomyopathy. Human Pathology, 46(11), 1760–1768.
Yancey, D. M., Guichard, J. L., Ahmed, M. I., Zhou, L., Murphy, M. P., Johnson, M. S., et al. (2015). Cardiomyocyte mitochondrial oxidative stress and cytoskeletal breakdown in the heart with a primary volume overload. American Journal of Physiology Heart and Circulatory Physiology, 308(6), H651–H663.
Chiong, M., Wang, Z. V., Pedrozo, Z., Cao, D. J., Troncoso, R., Ibacache, M., et al. (2011). Cardiomyocyte death: Mechanisms and translational implications. Cell Death and Disease, 2, e244.
Ha, M., & Kim, V. N. (2014). Regulation of microRNA biogenesis. Nature Reviews Molecular Cell Biology, 15(8), 509–524.
Chen, J., & Wang, D. Z. (2012). microRNAs in cardiovascular development. Journal of Molecular and Cellular Cardiology, 52(5), 949–957.
Dong, S., Cheng, Y., Yang, J., Li, J., Liu, X., Wang, X., et al. (2009). MicroRNA expression signature and the role of microRNA-21 in the early phase of acute myocardial infarction. Journal of Biological Chemistry, 284(43), 29514–29525.
Ishikawa, M., Tanaka, S., Arai, M., Genda, Y., & Sakamoto, A. (2012). Differences in microRNA changes of healthy rat liver between sevoflurane and propofol anesthesia. Anesthesiology, 117(6), 1245–1252.
Cao, L., Feng, C., Li, L., & Zuo, Z. (2012). Contribution of microRNA-203 to the isoflurane preconditioning-induced neuroprotection. Brain Research Bulletin, 88(5), 525–528.
Tanaka, S., Ishikawa, M., Arai, M., Genda, Y., & Sakamoto, A. (2012). Changes in microRNA expression in rat lungs caused by sevoflurane anesthesia: A TaqMan(R) low-density array study. Biomedical Research, 33(5), 255–263.
Olson, J. M., Yan, Y., Bai, X., Ge, Z. D., Liang, M., Kriegel, A. J., et al. (2015). Up-regulation of microRNA-21 mediates isoflurane-induced protection of cardiomyocytes. Anesthesiology, 122(4), 795–805.
Louch, W. E., Sheehan, K. A., & Wolska, B. M. (2011). Methods in cardiomyocyte isolation, culture, and gene transfer. Journal of Molecular and Cellular Cardiology, 51(3), 288–298.
Li, J., Aung, L. H., Long, B., Qin, D., An, S., & Li, P. (2015). miR-23a binds to p53 and enhances its association with miR-128 promoter. Scientific Reports, 5, 16422.
Cheng, Y., Zhang, R., Yang, G., Zhang, Y., Li, C., Zhang, M. et al. (2017). Mir-23a inhibition attenuates ischemic/reperfusion-induced myocardial apoptosis by targeting XIAP. International Journal of Clinical and Experimental Pathology, 10(10), 10374–10382.
Hu, Y., Deng, H., Xu, S., & Zhang, J. (2015). MicroRNAs regulate mitochondrial function in cerebral ischemia-reperfusion injury. International Journal of Molecular Sciences, 16(10), 24895–24917.
Lin, H., Qian, J., Castillo, A. C., Long, B., Keyes, K. T., Chen, G., et al. (2011). Effect of miR-23 on oxidant-induced injury in human retinal pigment epithelial cells. Investigative Ophthalmology and Visual Science, 52(9), 6308–6314.
Ilkun, O., & Boudina, S. (2013). Cardiac dysfunction and oxidative stress in the metabolic syndrome: An update on antioxidant therapies. Current Pharmaceutical Design, 19(27), 4806–4817.
Lee, Y. M., Song, B. C., & Yeum, K. J. (2015). Impact of volatile anesthetics on oxidative stress and inflammation. BioMed Research International, 2015, 242709.
Dringen, R. (2000). Metabolism and functions of glutathione in brain. Progress in Neurobiology, 62(6), 649–671.
Hashem, S. I., Perry, C. N., Bauer, M., Han, S., Clegg, S. D., Ouyang, K., et al. (2015). Brief report: Oxidative stress mediates cardiomyocyte apoptosis in a human model of danon disease and heart failure. Stem Cells, 33(7), 2343–2350.
Aikawa, R., Nawano, M., Gu, Y., Katagiri, H., Asano, T., Zhu, W., et al. (2000). Insulin prevents cardiomyocytes from oxidative stress-induced apoptosis through activation of PI3 kinase/Akt. Circulation, 102(23), 2873–2879.
Zhao, W., Fan, G. C., Zhang, Z. G., Bandyopadhyay, A., Zhou, X., & Kranias, E. G. (2009). Protection of peroxiredoxin II on oxidative stress-induced cardiomyocyte death and apoptosis. Basic Research in Cardiology, 104(4), 377–389.
Hirata, N., Shim, Y. H., Pravdic, D., Lohr, N. L., Pratt, P. F., Jr., Weihrauch, D., et al. (2011). Isoflurane differentially modulates mitochondrial reactive oxygen species production via forward versus reverse electron transport flow: Implications for preconditioning. Anesthesiology, 115(3), 531–540.
Donadelli, M., Dando, I., Fiorini, C., & Palmieri, M. (2014). Regulation of miR-23b expression and its dual role on ROS production and tumour development. Cancer Letters, 349(2), 107–113.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Rights and permissions
About this article
Cite this article
Liu, Hj., Liu, B. Inhibition of MicroRNA-23 Contributes to the Isoflurane-Mediated Cardioprotection Against Oxidative Stress. Cardiovasc Toxicol 18, 450–458 (2018). https://doi.org/10.1007/s12012-018-9455-1
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12012-018-9455-1