Abstract
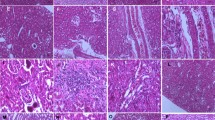
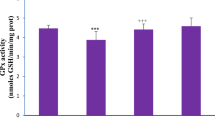
The objective of the present study was to examine the effects of long-term exposure on oxidative damage, Keap1/Nrf2 signaling pathway, and Msr-related redox status in the kidneys of rats. Therefore, in this experimental study, a total of 32 CD-1 rats were randomized into 4 groups and treated with 30-, 60-, and 120-mg/kg Cu for 24 weeks. Different serum biomarkers suggestive of renal functions, pathological changes, and oxidative stress were analyzed in kidney tissues. Moreover, the levels of the Keap1/Nrf2 signaling pathway and redox status–related gene mRNA and proteins were also detected. The results indicated that Cu exposure dramatically increased the contents of creatinine and carbamide. Furthermore, histopathological alterations and mitochondrial damage in kidneys of rats of different Cu-treated groups were obviously observed. In addition, Cu exposure markedly changed the levels of glutathione, catalase, and total antioxidant capacity, and upregulated the contents of protein carbonyl, nitric oxide, and malondialdehyde. Moreover, higher levels of Cu treatments significantly increased the expression of Keap1/Nrf2 signaling pathway and redox status–related genes (NQO1, SOD-1, TRX, MsrA, MsrB1, MsrB2, MsrB3). Simultaneously, the mRNA expression levels of Nrf2, HO-1, and CAT were upregulated in rats exposed to 30- and 60-mg/kg Cu, but downregulated in the 120-mg/kg Cu group compared with the control group. Moreover, the Keap1/Nrf2 signaling pathway and redox status–related protein expression levels (HO-1, SOD-1, TRX, MsrA, MsrB1, MsrB2) were significantly increased in treated rats. In summary, it is suggested that the Keap1/Nrf2 signaling pathway and activation of Msr prevent Cu-induced nephrotoxicity and attenuate oxidative damage.







Similar content being viewed by others
References
Wan F, Zhong G, Ning Z, Liao J, Yu W, Wang C, Han Q, Li Y, Pan J, Tang Z, Huang R, Hu L (2020) Long-term exposure to copper induces autophagy and apoptosis through oxidative stress in rat kidneys. Ecotoxicol Environ Saf 190:110158. https://doi.org/10.1016/j.ecoenv.2019.110158
Hill GM, Shannon MC (2019) Copper and zinc nutritional issues for agricultural animal production. Biol Trace Elem Res 188(1):148–159. https://doi.org/10.1007/s12011-018-1578-5
Adrees M, Ali S, Rizwan M, Ibrahim M, Abbas F, Farid M, Zia-Ur-Rehman M, Irshad MK, Bharwana SA (2015) The effect of excess copper on growth and physiology of important food crops: a review. Environ Sci Pollut Res Int 22(11):8148–8162. https://doi.org/10.1007/s11356-015-4496-5
Padrilah SN, Ahmad SA, Yasid NA, Sabullah MK, Daud HM, Khalid A, Shukor MY (2017) Toxic effects of copper on liver and cholinesterase of Clarias gariepinus. Environ Sci Pollut Res Int 24(28):22510–22523. https://doi.org/10.1007/s11356-017-9923-3
Pal A (2014) Copper toxicity induced hepatocerebral and neurodegenerative diseases: an urgent need for prognostic biomarkers. Neurotoxicology 40:97–101. https://doi.org/10.1016/j.neuro.2013.12.001
Shahzad MN, Javed MT, Shabir S, Irfan M, Hussain R (2012) Effects of feeding urea and copper sulphate in different combinations on live body weight, carcass weight, percent weight to body weight of different organs and histopathological tissue changes in broilers. Exp Toxicol Pathol 64(3):141–147. https://doi.org/10.1016/j.etp.2010.07.009
Khushboo M, Murthy MK, Devi MS, Sanjeev S, Ibrahim KS, Kumar NS, Roy VK, Gurusubramanian G (2018) Testicular toxicity and sperm quality following copper exposure in Wistar albino rats: ameliorative potentials of L-carnitine. Environ Sci Pollut Res 25(2):1837–1862. https://doi.org/10.1007/s11356-017-0624-8
Xu M, Tang H, Zhou X, Chen H, Dong Q, Zhang Y, Ye G, Shi F, Lv C, Jing B, He C, Zhao L, Li Y (2018) Effects and mechanisms of sub-chronic exposure to copper nanoparticles on renal cytochrome P450 enzymes in rats. Environ Toxicol Pharmacol 63:135–146. https://doi.org/10.1016/j.etap.2018.08.004
Gaetke LM, Chow CK (2003) Copper toxicity, oxidative stress, and antioxidant nutrients. Toxicology 189(1-2):147–163. https://doi.org/10.1016/s0300-483x(03)00159-8
Sun X, Li J, Zhao H, Wang Y, Liu J, Shao Y, Xue Y, Xing M (2018) Synergistic effect of copper and arsenic upon oxidative stress, inflammation and autophagy alterations in brain tissues of Gallus gallus. J Inorg Biochem 178:54–62. https://doi.org/10.1016/j.jinorgbio.2017.10.006
Pisoschi AM, Pop A (2015) The role of antioxidants in the chemistry of oxidative stress: a review. Eur J Med Chem 97:55–74. https://doi.org/10.1016/j.ejmech.2015.04.040
Ercal N, Gurer-Orhan H, Aykin-Burns N (2001) Toxic metals and oxidative stress part I: mechanisms involved in metal-induced oxidative damage. Curr Top Med Chem 1(6):529–539. https://doi.org/10.2174/1568026013394831
Yang F, Liao J, Pei R, Yu W, Han Q, Li Y, Guo J, Hu L, Pan J, Tang Z (2018) Autophagy attenuates copper-induced mitochondrial dysfunction by regulating oxidative stress in chicken hepatocytes. Chemosphere 204:36–43. https://doi.org/10.1016/j.chemosphere.2018.03.192
Ermakov AV, Konkova MS, Kostyuk SV, Izevskaya VL, Baranova A, Veiko NN (2013) Oxidized extracellular DNA as a stress signal in human cells. Oxidative Med Cell Longev 2013:649747–649747, 12. https://doi.org/10.1155/2013/649747
Chapple SJ, Siow RCM, Mann GE (2012) Crosstalk between Nrf2 and the proteasome: therapeutic potential of Nrf2 inducers in vascular disease and aging. Int J Biochem Cell Biol 44(8):1315–1320. https://doi.org/10.1016/j.biocel.2012.04.021
Chechushkov A, Zaitseva N, Vorontsova E, Kozhin P, Menshchikova E, Shkurupiy V (2016) Dextran loading protects macrophages from lipid peroxidation and induces a Keap1/Nrf2/ARE-dependent antioxidant response. Life Sci 166:100–107. https://doi.org/10.1016/j.lfs.2016.10.013
Hu J, Tian J, Zhang F, Wang H, Yin J (2019) Pxr- and Nrf2-mediated induction of ABC transporters by heavy metal ions in zebrafish embryos (Environ. Pollut.). Environ Pollut 255(Pt 2):113329–113329. https://doi.org/10.1016/j.envpol.2019.113329
Aladaileh SH, Hussein OE, Abukhalil MH, Saghir SAM, Bin-Jumah M, Alfwuaires MA, Germoush MO, Almaiman AA, Mahmoud AM (2019) Formononetin upregulates Nrf2/HO-1 signaling and prevents oxidative stress, inflammation, and kidney injury in methotrexate-induced rats. Antioxidants-Basel 8(10):430. https://doi.org/10.3390/antiox8100430
Li S, Jiang X, Luo Y, Zhou B, Shi M, Liu F, Sha A (2019) Sodium/calcium overload and Sirt1/Nrf2/OH-1 pathway are critical events in mercuric chloride-induced nephrotoxicity. Chemosphere 234:579–588. https://doi.org/10.1016/j.chemosphere.2019.06.095
Cabreiro F, Picot CR, Friguet B, Petropoulos I (2006) Methionine sulfoxide reductases: relevance to aging and protection against oxidative stress. Ann N Y Acad Sci 1067:37–44. https://doi.org/10.1196/annals.1354.006
Grimaud R, Ezraty B, Mitchell JK, Lafitte D, Briand C, Derrick PJ, Barras F (2001) Repair of oxidized proteins. Identification of a new methionine sulfoxide reductase. J Biol Chem 276(52):48915–48920. https://doi.org/10.1074/jbc.M105509200
Nan C, Li Y, Jean-Charles P-Y, Chen G, Kreymerman A, Prentice H, Weissbach H, Huang X (2010) Deficiency of methionine sulfoxide reductase A causes cellular dysfunction and mitochondrial damage in cardiac myocytes under physical and oxidative stresses. Biochem Biophys Res Commun 402(4):608–613. https://doi.org/10.1016/j.bbrc.2010.10.064
Noh MR, Kim KY, Han SJ, Kim JI, Kim H-Y, Park KM (2017) Methionine sulfoxide reductase A deficiency exacerbates cisplatin-induced nephrotoxicity via increased mitochondrial damage and renal cell death. Antioxid Redox Signal 27(11):727–741. https://doi.org/10.1089/ars.2016.6874
Ge J, Zhang C, Sun Y-C, Zhang Q, Lv M-W, Guo K, Li J-L (2019) Cadmium exposure triggers mitochondrial dysfunction and oxidative stress in chicken (Gallus gallus) kidney via mitochondrial UPR inhibition and Nrf2-mediated antioxidant defense activation. Sci Total Environ 689:1160–1171. https://doi.org/10.1016/j.scitotenv.2019.06.405
Ali S, Hussain S, Khan R, Mumtaz S, Ashraf N, Andleeb S, Shakir HA, Tahir HM, Khan MKA, Ulhaq M (2019) Renal toxicity of heavy metals (cadmium and mercury) and their amelioration with ascorbic acid in rabbits. Environ Sci Pollut Res Int 26(4):3909–3920. https://doi.org/10.1007/s11356-018-3819-8
Eom SY, Yim DH, Huang M, Park CH, Kim GB, Yu SD, Choi BS, Park JD, Kim YD, Kim H (2020) Copper-zinc imbalance induces kidney tubule damage and oxidative stress in a population exposed to chronic environmental cadmium. Int Arch Occup Environ Health 93(3):337–344. https://doi.org/10.1007/s00420-019-01490-9
Poprac P, Jomova K, Simunkova M, Kollar V, Rhodes CJ, Valko M (2017) Targeting free radicals in oxidative stress-related human diseases. Trends Pharmacol Sci 38(7):592–607. https://doi.org/10.1016/j.tips.2017.04.005
Oleson BJ, Broniowska KA, Naatz A, Hogg N, Tarakanova VL, Corbett JA (2016) Nitric oxide suppresses β-cell apoptosis by inhibiting the DNA damage response. Mol Cell Biol 36(15):2067–2077. https://doi.org/10.1128/MCB.00262-16
Wang H-W, Zhang Y, Tan P-P, Jia L-S, Chen Y, Zhou B-H (2019) Mitochondrial respiratory chain dysfunction mediated by ROS is a primary point of fluoride-induced damage in Hepa1-6 cells. Environ Pollut 255(Pt 3):113359–113359. https://doi.org/10.1016/j.envpol.2019.113359
Wang C, Ning Z, Wan F, Huang R, Chao L, Kang Z, Yang F, Zhong G, Li Y, Pan J, Tang Z, Hu L (2019) Characterization of the cellular effects and mechanism of arsenic trioxide-induced hepatotoxicity in broiler chickens. Toxicol in Vitro 61:104629–104629. https://doi.org/10.1016/j.tiv.2019.104629
Han Y, Li C, Su M, Wang Z, Jiang N, Sun D (2017) Antagonistic effects of selenium on lead-induced autophagy by influencing mitochondrial dynamics in the spleen of chickens. Oncotarget 8(20):33725, 33735. https://doi.org/10.18632/oncotarget.16736
Liao J, Yang F, Chen H, Yu W, Han Q, Li Y, Hu L, Guo J, Pan J, Liang Z, Tang Z (2019) Effects of copper on oxidative stress and autophagy in hypothalamus of broilers. Ecotox Environ Safe 185:109710–109710. https://doi.org/10.1016/j.ecoenv.2019.109710
Bellezza I, Giambanco I, Minelli A, Donato R (2018) Nrf2-Keap1 signaling in oxidative and reductive stress. BBA-Mol Cell Res 1865(5):721–733. https://doi.org/10.1016/j.bbamcr.2018.02.010
Cebula M, Schmidt EE, Arnér ESJ (2015) TrxR1 as a potent regulator of the Nrf2-Keap1 response system. Antioxid Redox Signal 23(10):823–853. https://doi.org/10.1089/ars.2015.6378
Sarkar S, Mukherjee S, Chattopadhyay A, Bhattacharya S (2014) Low dose of arsenic trioxide triggers oxidative stress in zebrafish brain: expression of antioxidant genes. Ecotox Environ Safe 107:1–8. https://doi.org/10.1016/j.ecoenv.2014.05.012
Kumar V, Kalita J, Bora HK, Misra UK (2016) Relationship of antioxidant and oxidative stress markers in different organs following copper toxicity in a rat model. Toxicol Appl Pharmacol 293:37–43. https://doi.org/10.1016/j.taap.2016.01.007
Yang D, Lv Z, Zhang H, Liu B, Jiang H, Tan X, Lu J, Baiyun R, Zhang Z (2017) Activation of the Nrf2 signaling pathway involving KLF9 plays a critical role in allicin resisting against arsenic trioxide-induced hepatotoxicity in rats. Biol Trace Elem Res 176(1):192–200. https://doi.org/10.1007/s12011-016-0821-1
Zhu H, Itoh K, Yamamoto M, Zweier JL, Li Y (2005) Role of Nrf2 signaling in regulation of antioxidants and phase 2 enzymes in cardiac fibroblasts: protection against reactive oxygen and nitrogen species-induced cell injury. FEBS Lett 579(14):3029–3036. https://doi.org/10.1016/j.febslet.2005.04.058
Zhang X-H, Weissbach H (2008) Origin and evolution of the protein-repairing enzymes methionine sulphoxide reductases. Biol Rev Camb Philos Soc 83(3):249–257. https://doi.org/10.1111/j.1469-185X.2008.00042.x
Lourenço Dos Santos S, Petropoulos I, Friguet B (2018) The oxidized protein repair enzymes methionine sulfoxide reductases and their roles in protecting against oxidative stress, in ageing and in regulating protein function. Antioxidants (Basel) 7(12):191. https://doi.org/10.3390/antiox7120191
Guan X-L, Wu P-F, Wang S, Zhang J-J, Shen Z-C, Luo H, Chen H, Long L-H, Chen J-G, Wang F (2017) Dimethyl sulfide protects against oxidative stress and extends lifespan via a methionine sulfoxide reductase A-dependent catalytic mechanism. Aging Cell 16(2):226–236. https://doi.org/10.1111/acel.12546
Brunell D, Weissbach H, Hodder P, Brot N (2010) A high-throughput screening compatible assay for activators and inhibitors of methionine sulfoxide reductase A. Assay Drug Dev Technol 8(5):615–620. https://doi.org/10.1089/adt.2009.0263
Weissbach H, Resnick L, Brot N (2005) Methionine sulfoxide reductases: history and cellular role in protecting against oxidative damage. Biochim Biophys Acta-Biomembr 1703(2):203–212. https://doi.org/10.1016/j.bbapap.2004.10.004
Marchetti MA, Lee W, Cowell TL, Wells TM, Weissbach H, Kantorow M (2006) Silencing of the methionine sulfoxide reductase A gene results in loss of mitochondrial membrane potential and increased ROS production in human lens cells. Exp Eye Res 83(5):1281–1286. https://doi.org/10.1016/j.exer.2006.07.005
Kim J-Y, Kim Y, Kwak G-H, Oh SY, Kim H-Y (2014) Over-expression of methionine sulfoxide reductase A in the endoplasmic reticulum increases resistance to oxidative and ER stresses. Acta Biochim Biophys Sin Shanghai 46(5):415–419. https://doi.org/10.1093/abbs/gmu011
Kaya A, Lee BC, Gladyshev VN (2015) Regulation of protein function by reversible methionine oxidation and the role of selenoprotein MsrB1. Antioxid Redox Signal 23(10):814–822. https://doi.org/10.1089/ars.2015.6385
Kim KY, Kwak G-H, Singh MP, Gladyshev VN, Kim H-Y (2017) Selenoprotein MsrB1 deficiency exacerbates acetaminophen-induced hepatotoxicity via increased oxidative damage. Arch Biochem Biophys 634:69–75. https://doi.org/10.1016/j.abb.2017.09.020
Kwak G-H, Kim T-H, Kim H-Y (2017) Down-regulation of MsrB3 induces cancer cell apoptosis through reactive oxygen species production and intrinsic mitochondrial pathway activation. Biochem Biophys Res Commun 483(1):468–474. https://doi.org/10.1016/j.bbrc.2016.12.120
Kwak G-H, Kim H-Y (2017) MsrB3 deficiency induces cancer cell apoptosis through p53-independent and ER stress-dependent pathways. Arch Biochem Biophys 621:1–5. https://doi.org/10.1016/j.abb.2017.04.001
Kim J-Y, Choi SH, Lee E, Kang YJ, Kim H-Y (2012) Methionine sulfoxide reductase A attenuates heme oxygenase-1 induction through inhibition of Nrf2 activation. Arch Biochem Biophys 528(2):134–140. https://doi.org/10.1016/j.abb.2012.09.012
Kwak G-H, Kim KY, Kim H-Y (2016) Methionine sulfoxide reductase B3 deficiency stimulates heme oxygenase-1 expression via ROS-dependent and Nrf2 activation pathways. Biochem Biophys Res Commun 473(4):1033–1038. https://doi.org/10.1016/j.bbrc.2016.04.011
Funding
This work was supported by the National Natural Science Foundation of China (#31402264 and #31572585) and the Guangzhou Science and Technology Program key projects (#201803020003).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
ESM 1
(DOCX 16 kb)
Rights and permissions
About this article
Cite this article
Zhong, G., He, Y., Wan, F. et al. Effects of Long-Term Exposure to Copper on the Keap1/Nrf2 Signaling Pathway and Msr-Related Redox Status in the Kidneys of Rats. Biol Trace Elem Res 199, 4205–4217 (2021). https://doi.org/10.1007/s12011-020-02557-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-020-02557-2