Abstract
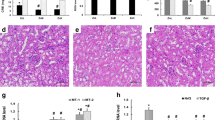
Zinc (Zn) is an important trace element in the body that has antioxidant effects. It has been proven that Zn deficiency can cause oxidative stress. The purpose of the present study was to clarify the effect and mechanism of Zn deficiency on myocardial fibrosis. Mice were fed with different Zn levels dietary for 9 weeks: Zn-normal group (ZnN, 34 mg Zn/kg), Zn-deficient group (ZnD, 2 mg Zn/kg), and Zn-adequate group (ZnA, 100 mg Zn/kg). We found that the Zn-deficient diet reduced the Zn concentration in myocardial tissue. Moreover, the TUNEL results demonstrated that cardiomyocytes in the ZnD group died in large numbers. Furthermore, ROS levels were significantly increased, and metallothionein (MT) expression levels decreased in the ZnD group. The results of Sirius Red staining indicated an increase in collagen in the ZnD group. Moreover, the ELISA results showed that collagen I, III, and IV and fibronectin (FN) were increased. In addition, the expression of matrix metalloproteinases (MMPs) and tissue inhibitors of metalloproteinase (TIMPs) was detected by RT-qPCR. The results showed that the expression of TIMP-1 in the ZnD group was increased, while MMPs were decreased. Immunohistochemical results showed an increase in the content of α-smooth muscle actin (α-SMA), while H&E staining showed an increase in interstitial width and a decrease in the number of cardiac cells. All data suggest that Zn deficiency enhances the oxidative stress response of myocardial tissue and eventually triggers myocardial fibrosis.





Similar content being viewed by others
References
Writing C, Smith SC Jr, Collins A, Ferrari R, Holmes DR Jr, Logstrup S et al (2012) Our time: a call to save preventable death from cardiovascular disease (heart disease and stroke). Glob Heart 7:297–305
Gyongyosi M, Winkler J, Ramos I, Do QT, Firat H, McDonald K et al (2017) Myocardial fibrosis: biomedical research from bench to bedside. Eur J Heart Fail 19:177–191
Weber KT, Sun Y, Bhattacharya SK, Ahokas RA, Gerling IC (2013) Myofibroblast-mediated mechanisms of pathological remodelling of the heart. Nat Rev Cardiol 10:15–26
Cohn JN, Ferrari R, Sharpe N (2000) Cardiac remodeling—concepts and clinical implications: a consensus paper from an international forum on cardiac remodeling. J Am Coll Cardiol 35:569–582
Gonzalez A, Ravassa S, Beaumont J, Lopez B, Diez J (2011) New targets to treat the structural remodeling of the myocardium. J Am Coll Cardiol 58:1833–1843
Zhao S, Wu H, Xia W, Chen X, Zhu S, Zhang S, Shao Y, Ma W, Yang D, Zhang J (2014) Periostin expression is upregulated and associated with myocardial fibrosis in human failing hearts. J Cardiol 63:373–378
Li AH, Liu PP, Villarreal FJ, Garcia RA (2014) Dynamic changes in myocardial matrix and relevance to disease: translational perspectives. Circ Res 114:916–927
Xiang FL, Fang M, Yutzey KE (2017) Loss of beta-catenin in resident cardiac fibroblasts attenuates fibrosis induced by pressure overload in mice. Nat Commun 8:712
Zebrowski DC, Engel FB (2013) The cardiomyocyte cell cycle in hypertrophy, tissue homeostasis, and regeneration. Rev Physiol Biochem Pharmacol 165:67–96
Kuhn B, del Monte F, Hajjar RJ, Chang YS, Lebeche D, Arab S et al (2007) Periostin induces proliferation of differentiated cardiomyocytes and promotes cardiac repair. Nat Med 13:962–969
Shamhart PE, Meszaros JG (2010) Non-fibrillar collagens: key mediators of post-infarction cardiac remodeling? J Mol Cell Cardiol 48:530–537
Nagase H, Visse R, Murphy G (2006) Structure and function of matrix metalloproteinases and TIMPs. Cardiovasc Res 69:562–573
Visse R, Nagase H (2003) Matrix metalloproteinases and tissue inhibitors of metalloproteinases: structure, function, and biochemistry. Circ Res 92:827–839
Scrimgeour AG, Carrigan CT, Condlin ML, Urso ML, van den Berg RM, van Helden HPM, Montain SJ, Joosen MJA (2018) Dietary zinc modulates matrix metalloproteinases in traumatic brain injury. J Neurotrauma 35:2495–2506
Yoshida A, Kanamori H, Naruse G, Minatoguchi S, Iwasa M, Yamada Y, Mikami A, Kawasaki M, Nishigaki K, Minatoguchi S (2019) (Pro)renin receptor blockade ameliorates heart failure caused by chronic kidney disease. J Card Fail 25:286–300
Jarosz M, Olbert M, Wyszogrodzka G, Mlyniec K, Librowski T (2017) Antioxidant and anti-inflammatory effects of zinc. Zinc-dependent NF-kappaB signaling. Inflammopharmacology 25:11–24
Vasak M, Meloni G (2011) Chemistry and biology of mammalian metallothioneins. J Biol Inorganic Chem : JBIC : a publication of the Society of Biological Inorganic Chemistry 16:1067–1078
Ji SG, Weiss JH (2018) Zn2+-induced disruption of neuronal mitochondrial function: synergism with Ca2+, critical dependence upon cytosolic Zn2+ buffering, and contributions to neuronal injury. Exp Neurol 302:181–195
Castro L, Freeman B (2001) Reactive oxygen species in human health and disease. Nutrition 17:161–165
Lorenzen JM, Schauerte C, Hubner A, Kolling M, Martino F, Scherf K et al (2015) Osteopontin is indispensible for AP1-mediated angiotensin II-related miR-21 transcription during cardiac fibrosis. Eur Heart J 36:2184–2196
Xiang FL, Guo MZ, Yutzey KE (2016) Overexpression of Tbx20 in adult cardiomyocytes promotes proliferation and improves cardiac function after myocardial infarction. Circulation 133:1081–1092
Tomat AL, Costa MD, Arranz CT (2011) Zinc restriction during different periods of life: influence in renal and cardiovascular diseases. Nutrition 27:392–398
Maret W (2000) The function of zinc metallothionein: a link between cellular zinc and redox state. J Nutr 130:1455s–1458s
Oliveira HCF, Cosso RG, Alberici LC, Maciel EN, Salerno AG, Dorighello GG et al (2004) Oxidative stress in atherosclerosis-prone mouse is due to low antioxidant capacity of mitochondria. FASEB J 18:278
Sato M, Kondoh M (2002) Recent studies on metallothionein: protection against toxicity of heavy metals and oxygen free radicals. Tohoku J Exp Med 196:9–22
Yang LF, Ma JP, Tan Y, Zheng QJ, Dong ML, Guo W et al (2019) Cardiac-specific overexpression of metallothionein attenuates L-NAME-induced myocardial contractile anomalies and apoptosis. J Cell Mol Med 23:4640–4652
Laity JH, Andrews GK (2007) Understanding the mechanisms of zinc-sensing by metal-response element binding transcription factor-1 (MTF-1). Arch Biochem Biophys 463:201–210
Prasad AS (2008) Zinc in human health: effect of zinc on immune cells. Mol Med 14:353–357
Frangogiannis NG (2019) Cardiac fibrosis: cell biological mechanisms, molecular pathways and therapeutic opportunities. Mol Asp Med 65:70–99
Li L, Zhao Q, Kong W (2018) Extracellular matrix remodeling and cardiac fibrosis. Matrix Biol 68-69:490–506
Malemud CJ (2006) Matrix metalloproteinases (MMPs) in health and disease: an overview. Front Biosci-Landmrk 11:1696–1701
Peterson JT, Li H, Dillon L, Bryant JW (2000) Evolution of matrix metalloprotease and tissue inhibitor expression during heart failure progression in the infarcted rat. Cardiovasc Res 46:307–315
Lovelock JD, Baker AH, Gao F, Dong JF, Bergeron AL, McPheat W, Sivasubramanian N, Mann DL (2005) Heterogeneous effects of tissue inhibitors of matrix metalloproteinases on cardiac fibroblasts. Am J Physiol-Heart C 288:H461–H468
Karamanos NK, Theocharis AD, Neill T, Iozzo RV (2019) Matrix modeling and remodeling: a biological interplay regulating tissue homeostasis and diseases. Matrix Biol 75-76:1–11
Barbolina MV, Stack MS (2008) Membrane type 1-matrix metalloproteinase: substrate diversity in pericellular proteolysis. Semin Cell Dev Biol 19:24–33
Moore L, Fan D, Basu R, Kandalam V, Kassiri Z (2012) Tissue inhibitor of metalloproteinases (TIMPs) in heart failure. Heart Fail Rev 17:693–706
Sivasubramanian N, Coker ML, Kurrelmeyer KM, MacLellan WR, DeMayo FJ, Spinale FG et al (2001) Left ventricular remodeling in transgenic mice with cardiac restricted overexpression of tumor necrosis factor. Circulation 104:826–831
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
All animals were obtained from Huazhong Agricultural University Laboratory Animal Research Center (Wuhan, China), according to the animal welfare standards of use, and approved by the Ethical Committee on Animal Research at Huazhong Agricultural University (HZAUMO-2015-12).
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Cao, Jw., Duan, Sy., Zhang, Hx. et al. Zinc Deficiency Promoted Fibrosis via ROS and TIMP/MMPs in the Myocardium of Mice. Biol Trace Elem Res 196, 145–152 (2020). https://doi.org/10.1007/s12011-019-01902-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-019-01902-4