Abstract
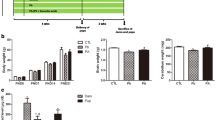
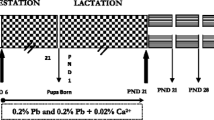
We evaluated the effect of lead (Pb) and ascorbic acid treatment of pregnant female rats on cerebellar development in pups. Pb was administered in drinking water (0.2% Pb acetate), and ascorbic acid (100 mg/kg) was administered through oral intubation. Fifteen female rats were randomly classified into control, Pb, and Pb plus ascorbic acid (PA) groups. The treatment of Pb and ascorbic acid treatments were terminated after birth to evaluate the effects on the gestational development of the cerebellum. At postnatal day 21 (PND21), pups were sacrificed, and blood Pb level was analyzed. Blood Pb levels of pups and dams were highest in the Pb group and reduced in the PA group. Immunohistochemistry and immunoblot assays were conducted to study the cerebellar expression levels of synaptic proteins. Along with a significant reduction in Purkinje cells, the reduction in presynaptic (synaptophysin) and postsynaptic (postsynaptic density protein 95, N-methyl-d-aspartate receptor subtype 1) marker proteins was observed in Pb-exposed pups. Ascorbic acid treatment significantly prevented Pb-induced impairment in the cerebellar synaptic proteins. Hypothesizing that brain-derived neurotrophic factor (BDNF) might be affected by Pb exposure given its importance in the regulation of synaptogenesis, we observed a Pb-induced decrease and ascorbic acid-mediated increase of BDNF in the cerebellum. Luxol fast blue staining and myelin basic protein analysis suggest that ascorbic acid treatment ameliorated the Pb exposure-induced reduction in the axonal fibers in the developing cerebellum. Overall, we conclude that ascorbic acid treatment during pregnancy can prevent Pb-induced impairments in the cerebellar development in rats.





Similar content being viewed by others
References
World Health Organization (2010) Childhood lead poisoning http://www.who.int/ceh/publications/childhoodpoisoning/en
Boucher O, Muckle G, Jacobson JL, Carter RC, Kaplan-Estrin M, Ayotte P, Dewailly É, Jacobson SW (2014) Domain-specific effects of prenatal exposure to PCBs, mercury, and lead on infant cognition: results from the Environmental Contaminants and Child Development Study in Nunavik. Environ Health Perspect 122:310–316
Bellinger DC (2016) Lead contamination in flint—an abject failure to protect public health. N Engl J Med 374:1101–1103
Mielke HW, Gonzales CR, Powell ET (2017) Soil lead and children’s blood lead disparities in pre- and post-hurricane Katrina New Orleans (USA). Int J Environ Res Public Health 14:e407
Froehlich TE, Lanphear BP, Auinger P, Hornung R, Epstein JN, Braun J, Kahn RS (2009) Association of tobacco and lead exposures with attention-deficit/hyperactivity disorder. Pediatrics 124:e1054–e1063
Opler MG, Buka SL, Groeger J, McKeague I, Wei C, Factor-Litvak P, Bresnahan M, Graziano J, Goldstein JM, Seidman LJ, Brown AS, Susser ES (2008) Prenatal exposure to lead, delta-aminolevulinic acid, and schizophrenia: further evidence. Environ Health Perspect 116:1586–1590
Wu J, Basha MR, Brock B, Cox DP, Cardozo-Pelaez F, McPherson CA, Harry J, Rice DC, Maloney B, Chen D, Lahiri DK, Zawia NH (2008) Alzheimer’s disease (AD)-like pathology in aged monkeys after infantile exposure to environmental metal lead (Pb): evidence for a developmental origin and environmental link for AD. J Neurosci 28:3–9
Wiedenmann B, Franke WW (1985) Identification and localization of synaptophysin, an integral memebrane glycoprotein of Mr 38,000 characteristic of presynaptic vesicles. Cell 41:1017–1028
Saito S, Kobayashi S, Ohashi Y, Igarashi M, Komiya Y, Ando S (1994) Decreased synaptic density in aged brains and its prevention by rearing under enriched environment as revealed by synaptophysin contents. J Neurosci Res 39:57–62
Valtorta F, Pennuto M, Bonanomi D, Benfenati F (2004) Synaptophysin: leading actor or walk-on role in synaptic vesicle exocytosis? BioEssays 26:445–453
Schwalm MT, Pasquali M, Miguel SP, Dos Santos JP, Vuolo F, Comim CM, Petronilho F, Quevedo J, Gelain DP, Moreira JC, Ritter C, Dal-Pizzol F (2014) Acute brain inflammation and oxidative damage are related to long-term cognitive deficits and markers of neurodegeneration in sepsis-survivor rats. Mol Neurobiol 49:380–385
Matosin N, Fernandez-Enright F, Lum JS, Engel M, Andrews JL, Gassen NC, Wagner KV, Schmidt MV, Newell KA (2016) Molecular evidence of synaptic pathology in the CA1 region in schizophrenia. NPJ Schizophr 2:16022
Wu DM, Lu J, Zheng YL, Zhou Z, Shan Q, Ma DF (2008) Purple sweet potato color repairs d-galactose-induced spatial learning and memory impairment by regulating the expression of synaptic proteins. Neurobiol Learn Mem 90:19–27
Yu H, Li T, Cui Y, Liao Y, Wang G, Gao L, Zhao F, Jin Y (2014) Effects of lead exposure on D-serine metabolism in the hippocampus of mice at the early developmental stages. Toxicology 325:189–199
Yu H, Liao Y, Li T, Cui Y, Wang G, Zhao F, Jin Y (2016) Alterations of synaptic proteins in the hippocampus of mouse offspring induced by developmental lead exposure. Mol Neurobiol 53:6786–6798
Holtzman D, DeVries C, Nguyen H, Olson J, Bensch K (1984) Maturation of resistance to lead encephalopathy: cellular and subcellular mechanisms. Neurotoxicology 5:97–124
Mousa AM, Al-Fadhli AS, Rao MS, Kilarkaje N (2015) Gestational lead exposure induces developmental abnormalities and up-regulates apoptosis of fetal cerebellar cells in rats. Drug Chem Toxicol 38:73–83
Nam SM, Ahn SC, Go TH, Seo JS, Nahm SS, Chang BJ, Lee JH (2018) Ascorbic acid ameliorates gestational lead exposure-induced developmental alteration in GAD67 and c-Kit expression in the rat cerebellar cortex. Biol Trace Elem Res 182:278–286
Chang BJ, Jang BJ, Son TG, Cho IH, Quan FS, Choe NH, Nahm SS, Lee JH (2012) Ascorbic acid ameliorates oxidative damage induced by maternal low-level lead exposure in the hippocampus of rat pups during gestation and lactation. Food Chem Toxicol 50:104–108
Goyer RA, Cherian MG (1979) Ascorbic acid and EDTA treatment of lead toxicity in rats. Life Sci 24:433–438
Needleman HL (1999) History of lead poisoning in the world. In: George AM (ed) Lead poisoning prevention and treatment in developing countries. The George Foundation, Bangalore, pp 17–25
Volk B (1984) Cerebellar histogenesis and synaptic maturation following pre- and postnatal alcohol administration. An electron-microscopic investigation of the rat cerebellar cortex. Acta Neuropathol 63:57–65
Nam SM, Chang BJ, Kim JH, Nahm SS, Lee JH (2018) Ascorbic acid ameliorates lead-induced apoptosis in the cerebellar cortex of developing rats. Brain Res 1686:10–18
Li J, Ma Y, Teng YD, Zheng K, Vartanian TK, Snyder EY, Sidman RL (2006) Purkinje neuron degeneration in nervous (nr) mutant mice is mediated by a metabolic pathway involving excess tissue plasminogen activator. Proc Natl Acad Sci U S A 103:7847–7852
Cohen-Cory S, Kidane AH, Shirkey NJ, Marshak S (2010) Brain-derived neurotrophic factor and the development of structural neuronal connectivity. Dev Neurobiol 70:271–288
Vazquez-Sanroman D, Leto K, Cerezo-Garcia M, Carbo-Gas M, Sanchis-Segura C, Carulli D, Rossi F, Miquel M (2015) The cerebellum on cocaine: plasticity and metaplasticity. Addict Biol 20:941–955
Hossain S, Bhowmick S, Jahan S, Rozario L, Sarkar M, Islam S, Basunia MA, Rahman A, Choudhury BK, Shahjalal H (2016) Maternal lead exposure decreases the levels of brain development and cognition-related proteins with concomitant upsurges of oxidative stress, inflammatory response and apoptosis in the offspring rats. Neurotoxicology 56:150–158
Aghajanian GK, Bloom FE (1967) The formation of synaptic junctions in developing rat brain: a quantitative electron microscopic study. Brain Res 6(4):716–727
Zimatkin SM, Karnyushko OA (2017) Synaptogenesis in the developing rat cerebellum. Neurosci Behav Physiol 47:631–636
McClatchy DB, Liao L, Park SK, Venable JD, Yates JR (2007) Quantification of the synaptosomal proteome of the rat cerebellum during post-natal development. Genome Res 17:1378–1388
Fujita M, Kadota T, Sato T (1996) Developmental profiles of synaptophysin in granule cells of rat cerebellum: an immunohistocytochemical study. J Electron Microsc 45:185–194
Leclerc N, Beesley PW, Brown I, Colonnier M, Gurd JW, Paladino T, Hawkes R (1989) Synaptophysin expression during synaptogenesis in the rat cerebellar cortex. J Comp Neurol 280:197–212
Fletcher TL, Cameron P, De Camilli P, Banker G (1991) The distribution of synapsin I and synaptophysin in hippocampal neurons developing in culture. J Neurosci 11:1617–1626
Gąssowska M, Baranowska-Bosiacka I, Moczydłowska J, Frontczak-Baniewicz M, Gewartowska M, Strużyńska L, Gutowska I, Chlubek D, Adamczyk A (2016) Perinatal exposure to lead (Pb) induces ultrastructural and molecular alterations in synapses of rat offspring. Toxicology 373:13–29
Sheng M (2001) The postsynaptic NMDA-receptor--PSD-95 signaling complex in excitatory synapses of the brain. J Cell Sci 114:1251
Steiner P, Higley MJ, Xu W, Czervionke BL, Malenka RC, Sabatini BL (2008) Destabilization of the postsynaptic density by PSD-95 serine 73 phosphorylation inhibits spine growth and synaptic plasticity. Neuron 60:788–802
Stansfield KH, Pilsner JR, Lu Q, Wright RO, Guilarte TR (2012) Dysregulation of BDNF-TrkB signaling in developing hippocampal neurons by Pb2+: implications for an environmental basis of neurodevelopmental disorders. Toxicol Sci 127:277–295
Alkondon M, Costa AC, Radhakrishnan V, Aronstam RS, Albuquerque EX (1990) Selective blockade of NMDA-activated channel currents may be implicated in learning deficits caused by lead. FEBS Lett 261:124–130
Neal AP, Worley PF, Guilarte TR (2011) Lead exposure during synaptogenesis alters NMDA receptor targeting via NMDA receptor inhibition. Neurotoxicology 32:281–289
Domith I, Socodato R, Portugal CC, Munis AF, Duarte-Silva AT, Paes-de-Carvalho R (2018) Vitamin C modulates glutamate transport and NMDA receptor function in the retina. J Neurochem 144:408–420
Grant MM, Barber VS, Griffiths HR (2005) The presence of ascorbate induces expression of brain derived neurotrophic factor in SH-SY5Y neuroblastoma cells after peroxide insult, which is associated with increased survival. Proteomics 5:534–540
Ashwin R, Sampath M, Gayathri R, Rajalakshmi R, Sudhanshu S (2013) A comparison of resveratrol and vitamin C therapy on expression of BDNF in stressed rat brain homogenate. IOSR J Pharm 10:22–27
Schwartz PM, Borghesani PR, Levy RL, Pomeroy SL, Segal RA (1997) Abnormal cerebellar development and foliation in BDNF−/− mice reveals a role for neurotrophins in CNS patterning. Neuron 19:269–281
Eldridge CF, Bunge MB, Bunge RP, Wood PM (1987) Differentiation of axon-related Schwann cells in vitro. I. Ascorbic acid regulates basal lamina assembly and myelin formation. J Cell Biol 105:1023–1034
Zhang L, Ma Z, Smith GM, Wen X, Pressman Y, Wood PM, Xu XM (2009) GDNF-enhanced axonal regeneration and myelination following spinal cord injury is mediated by primary effects on neurons. Glia 57:1178–1191
Kim JY, Choi CS, Hong SK (2014) Coculture of Schwann cells and neuronal cells for myelination in rat. Rapid Commun Photoscience 3:48–49
Contributions
All authors conceived and designed the study. SMN, JSS, and JHL conducted animal modeling and histological analysis. ISC, THG, and BJC conducted animal caring and immunoblot analysis. SMN, ISC, BJC, and JHL collected and analyzed the data and wrote the manuscript. JHK and SSN participated in designing of the study, discussing of results, and revising the manuscript. All authors approved the final version of the manuscript.
Funding
This research was supported by the faculty research fund of Konkuk University and the Veterinary Science Research Institute of Konkuk University.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Nam, S.M., Cho, IS., Seo, J.S. et al. Ascorbic Acid Attenuates Lead-Induced Alterations in the Synapses in the Developing Rat Cerebellum. Biol Trace Elem Res 187, 142–150 (2019). https://doi.org/10.1007/s12011-018-1354-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-018-1354-6