Abstract
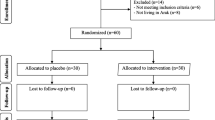
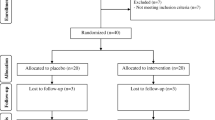
This study was carried out to investigate the effects of chromium intake on glycemic control, markers of cardio-metabolic risk, and oxidative stress in infertile polycystic ovary syndrome (PCOS) women candidate for in vitro fertilization (IVF). This randomized double-blind, placebo-controlled trial was done among 40 subjects with infertile PCOS candidate for IVF, aged 18–40 years old. Individuals were randomly allocated into two groups to take either 200 μg/day of chromium (n = 20) or placebo (n = 20) for 8 weeks. Biochemical parameters were assessed at baseline and at end-of-trial. Compared with the placebo, taking chromium supplements led to significant reductions in fasting plasma glucose (− 2.3 ± 5.7 vs. + 0.9 ± 3.1 mg/dL, P = 0.03), insulin levels (− 1.4 ± 2.1 vs. + 0.4 ± 1.7 μIU/mL, P = 0.004), homeostatic model of assessment for insulin resistance (− 0.3 ± 0.5 vs. + 0.1 ± 0.4, P = 0.005), and a significant increase in quantitative insulin sensitivity check index (+ 0.004 ± 0.008 vs. − 0.001 ± 0.008, P = 0.03). In addition, chromium supplementation significantly decreased serum triglycerides (− 19.2 ± 33.8 vs. + 8.3 ± 21.7 mg/dL, P = 0.004), VLDL- (− 3.8 ± 6.8 vs. + 1.7 ± 4.3 mg/dL, P = 0.004) and total cholesterol concentrations (− 15.3 ± 26.2 vs. − 0.6 ± 15.9 mg/dL, P = 0.03) compared with the placebo. Additionally, taking chromium supplements was associated with a significant increase in plasma total antioxidant capacity (+ 153.9 ± 46.1 vs. − 7.8 ± 43.9 mmol/L, P < 0.001) and a significant reduction in malondialdehyde values (−0.3 ± 0.3 vs. + 0.1 ± 0.2 μmol/L, P = 0.001) compared with the placebo. Overall, our study supported that chromium administration for 8 weeks to infertile PCOS women candidate for IVF had beneficial impacts on glycemic control, few variables of cardio-metabolic risk, and oxidative stress.

Similar content being viewed by others
Change history
28 February 2020
The Editors-in-Chief are currently investigating this article [Jamilian, M., Zadeh Modarres, S., Amiri Siavashani, M. et al. The Influences of Chromium Supplementation on Glycemic Control, Markers of Cardio-Metabolic Risk, and Oxidative Stress in Infertile Polycystic ovary Syndrome Women Candidate for In vitro Fertilization: a Randomized, Double-Blind, Placebo-Controlled Trial. Biol Trace Elem Res 185, 48–55 (2018). https://doi.org/10.1007/s12011-017-1236-3] as concerns have been raised about integrity of the clinical trial reported here. There is also an ongoing investigation by the Iranian National Committee for Ethics in Biomedical Researches. Further editorial action will be taken as appropriate once the investigation into the concerns is complete and all parties have been given an opportunity to respond in full.
References
Ehrmann DA (2005) Polycystic ovary syndrome. N Engl J Med 352(12):1223–1236. https://doi.org/10.1056/NEJMra041536
Hwang JL, Seow KM, Lin YH, Huang LW, Hsieh BC, Tsai YL, Wu GJ, Huang SC, Chen CY, Chen PH, Tzeng CR (2004) Ovarian stimulation by concomitant administration of cetrorelix acetate and HMG following Diane-35 pre-treatment for patients with polycystic ovary syndrome: a prospective randomized study. Hum Reprod 19(9):1993–2000. https://doi.org/10.1093/humrep/deh375
MacDougall MJ, Tan SL, Balen A, Jacobs HS (1993) A controlled study comparing patients with and without polycystic ovaries undergoing in-vitro fertilization. Hum Reprod 8(2):233–237. https://doi.org/10.1093/oxfordjournals.humrep.a138029
Brewer CJ, Balen AH (2010) The adverse effects of obesity on conception and implantation. Reproduction 140(3):347–364. https://doi.org/10.1530/REP-09-0568
Ramoglu S, Yoldemir T, Atasayan K, Yavuz DG (2017) Does cardiovascular risk vary according to the criteria for a diagnosis of polycystic ovary syndrome? J Obstet Gynaecol Res 43(12):1848–1854. https://doi.org/10.1111/jog.13455. [Epub ahead of print]
Cano F, Garcia-Velasco JA, Millet A, Remohi J, Simon C, Pellicer A (1997) Oocyte quality in polycystic ovaries revisited: identification of a particular subgroup of women. J Assist Reprod Genet 14(5):254–261. https://doi.org/10.1007/BF02765826
Vlaisavljevic V, Kovac V, Sajko MC (2009) Impact of insulin resistance on the developmental potential of immature oocytes retrieved from human chorionic gonadotropin-primed women with polycystic ovary syndrome undergoing in vitro maturation. Fertil Steril 91(3):957–959. https://doi.org/10.1016/j.fertnstert.2007.12.062
Ingle ME, Bloom MS, Parsons PJ, Steuerwald AJ, Kruger P, Fujimoto VY (2017) Associations between IVF outcomes and essential trace elements measured in follicular fluid and urine: a pilot study. J Assist Reprod Genet 34(2):253–261. https://doi.org/10.1007/s10815-016-0853-7
Vincent JB (2000) Elucidating a biological role for chromium at a molecular level. Acc Chem Res 33(7):503–510. https://doi.org/10.1021/ar990073r
Fraga CG (2005) Relevance, essentiality and toxicity of trace elements in human health. Mol Asp Med 26(4-5):235–244. https://doi.org/10.1016/j.mam.2005.07.013
Keen CL, Ensunsa JL, Watson MH, Baly DL, Donovan SM, Monaco MH, Clegg MS (1999) Nutritional aspects of manganese from experimental studies. Neurotoxicology 20(2-3):213–223
Fazelian S, Rouhani MH, Bank SS, Amani R (2017) Chromium supplementation and polycystic ovary syndrome: a systematic review and meta-analysis. J Trace Elem Med Biol 42:92–96. https://doi.org/10.1016/j.jtemb.2017.04.008
Jamilian M, Asemi Z (2015) Chromium supplementation and the effects on metabolic status in women with polycystic ovary syndrome: a randomized, double-blind, placebo-controlled trial. Ann Nutr Metab 67(1):42–48. https://doi.org/10.1159/000438465
Lucidi RS, Thyer AC, Easton CA, Holden AE, Schenken RS, Brzyski RG (2005) Effect of chromium supplementation on insulin resistance and ovarian and menstrual cyclicity in women with polycystic ovary syndrome. Fertil Steril 84(6):1755–1757. https://doi.org/10.1016/j.fertnstert.2005.06.028
Sahin K, Tuzcu M, Orhan C, Sahin N, Kucuk O, Ozercan IH, Juturu V, Komorowski JR (2013) Anti-diabetic activity of chromium picolinate and biotin in rats with type 2 diabetes induced by high-fat diet and streptozotocin. Br J Nutr 110(02):197–205. https://doi.org/10.1017/S0007114512004850
Martino F, Puddu PE, Pannarale G, Colantoni C, Martino E, Niglio T, Zanoni C, Barilla F (2013) Low dose chromium-polynicotinate or policosanol is effective in hypercholesterolemic children only in combination with glucomannan. Atherosclerosis 228(1):198–202. https://doi.org/10.1016/j.atherosclerosis.2013.02.005
Rotterdam ESHRE/ASRM-Sponsored PCOS Consensus Workshop Group (2004) Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome. Fertil Steril 81:19–25
Pisprasert V, Ingram KH, Lopez-Davila MF, Munoz AJ, Garvey WT (2013) Limitations in the use of indices using glucose and insulin levels to predict insulin sensitivity: impact of race and gender and superiority of the indices derived from oral glucose tolerance test in African Americans. Diabetes Care 36(4):845–853. https://doi.org/10.2337/dc12-0840
Benzie IF, Strain JJ (1996) The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: the FRAP assay. Anal Biochem 239(1):70–76. https://doi.org/10.1006/abio.1996.0292
Beutler E, Gelbart T (1985) Plasma glutathione in health and in patients with malignant disease. J Lab Clin Med 105(5):581–584
Janero DR (1990) Malondialdehyde and thiobarbituric acid-reactivity as diagnostic indices of lipid peroxidation and peroxidative tissue injury. Free Radic Biol Med 9(6):515–540. https://doi.org/10.1016/0891-5849(90)90131-2
Hatch R, Rosenfield RL, Kim MH, Tredway D (1981) Hirsutism: implications, etiology, and management. Am J Obstet Gynecol 140(7):815–830. https://doi.org/10.1016/0002-9378(81)90746-8
Asemi Z, Foroozanfard F, Hashemi T, Bahmani F, Jamilian M, Esmaillzadeh A (2015) Calcium plus vitamin D supplementation affects glucose metabolism and lipid concentrations in overweight and obese vitamin D deficient women with polycystic ovary syndrome. Clin Nutr 34(4):586–592. https://doi.org/10.1016/j.clnu.2014.09.015
Foroozanfard F, Jamilian M, Bahmani F, Talaee R, Talaee N, Hashemi T, Nasri K, Asemi Z, Esmaillzadeh A (2015) Calcium plus vitamin D supplementation influences biomarkers of inflammation and oxidative stress in overweight and vitamin D-deficient women with polycystic ovary syndrome: a randomized double-blind placebo-controlled clinical trial. Clin Endocrinol 83(6):888–894. https://doi.org/10.1111/cen.12840
Chakraborty P, Ghosh S, Goswami SK, Kabir SN, Chakravarty B, Jana K (2013) Altered trace mineral milieu might play an aetiological role in the pathogenesis of polycystic ovary syndrome. Biol Trace Elem Res 152(1):9–15. https://doi.org/10.1007/s12011-012-9592-5
Vincent JB (2017) New evidence against chromium as an essential trace element. J Nutr 147(12):2212–2219. https://doi.org/10.3945/jn.117.255901
Jeejeebhoy KN, Chu RC, Marliss EB, Greenberg GR, Bruce-Robertson A (1977) Chromium deficiency, glucose intolerance, and neuropathy reversed by chromium supplementation, in a patient receiving long-term total parenteral nutrition. Am J Clin Nutr 30(4):531–538
Saiyed ZM, Lugo JP (2016) Impact of chromium dinicocysteinate supplementation on inflammation, oxidative stress, and insulin resistance in type 2 diabetic subjects: an exploratory analysis of a randomized, double-blind, placebo-controlled study. Food Nutr Res 60(1):31762. https://doi.org/10.3402/fnr.v60.31762
Chen WY, Mao FC, Liu CH, Kuan YH, Lai NW, Wu CC, Chen CJ (2016) Chromium supplementation improved post-stroke brain infarction and hyperglycemia. Metab Brain Dis 31(2):289–297. https://doi.org/10.1007/s11011-015-9749-y
Chen TS, Chen YT, Liu CH, Sun CC, Mao FC (2015) Effect of chromium supplementation on element distribution in a mouse model of polycystic ovary syndrome. Biol Trace Elem Res 168(2):472–480. https://doi.org/10.1007/s12011-015-0384-6
Masharani U, Gjerde C, McCoy S, Maddux BA, Hessler D, Goldfine ID, Youngren JF (2012) Chromium supplementation in non-obese non-diabetic subjects is associated with a decline in insulin sensitivity. BMC Endocr Disord 12(1):31. https://doi.org/10.1186/1472-6823-12-31
Kelly CJ, Speirs A, Gould GW, Petrie JR, Lyall H, Connell JM (2002) Altered vascular function in young women with polycystic ovary syndrome. J Clin Endocrinol Metab 87(2):742–746. https://doi.org/10.1210/jcem.87.2.8199
Davis CM, Sumrall KH, Vincent JB (1996) A biologically active form of chromium may activate a membrane phosphotyrosine phosphatase (PTP). Biochemistry 35(39):12963–12969. https://doi.org/10.1021/bi960328y
Davis CM, Vincent JB (1997) Chromium oligopeptide activates insulin receptor tyrosine kinase activity. Biochemistry 36(15):4382–4385. https://doi.org/10.1021/bi963154t
Wang ZQ, Zhang XH, Russell JC, Hulver M, Cefalu WT (2006) Chromium picolinate enhances skeletal muscle cellular insulin signaling in vivo in obese, insulin-resistant JCR:LA-cp rats. J Nutr 136(2):415–420
Huang H, Chen G, Dong Y, Zhu Y, Chen H (2017) Chromium supplementation for adjuvant treatment of type 2 diabetes mellitus: results from a pooled analysis. Mol Nutr Food Res. https://doi.org/10.1002/mnfr.201700438. [Epub ahead of print]
Xiao F, Ao D, Zhou B, Spears JW, Lin X, Huang Y (2017) Effects of supplemental chromium propionate on serum lipids, carcass traits, and meat quality of heat-stressed broilers. Biol Trace Elem Res 176(2):401–406. https://doi.org/10.1007/s12011-016-0852-7
Rabinovitz H, Friedensohn A, Leibovitz A, Gabay G, Rocas C, Habot B (2004) Effect of chromium supplementation on blood glucose and lipid levels in type 2 diabetes mellitus elderly patients. Int J Vitam Nutr Res 74(3):178–182. https://doi.org/10.1024/0300-9831.74.3.178
Paiva AN, Lima JG, Medeiros AC, Figueiredo HA, Andrade RL, Ururahy MA, Rezende AA, Brandao-Neto J, Almeida M (2015) Beneficial effects of oral chromium picolinate supplementation on glycemic control in patients with type 2 diabetes: a randomized clinical study. J Trace Elem Med Biol 32:66–72. https://doi.org/10.1016/j.jtemb.2015.05.006
Li S, Chu Q, Ma J, Sun Y, Tao T, Huang R, Liao Y, Yue J, Zheng J, Wang L, Xue X, Zhu M, Kang X, Yin H, Liu W (2017) Discovery of novel lipid profiles in pcos: do insulin and androgen oppositely regulate bioactive lipid production? J Clin Endocrinol Metab 102(3):810–821. https://doi.org/10.1210/jc.2016-2692
Zhou B, Wang H, Luo G, Niu R, Wang J (2013) Effect of dietary yeast chromium and L-carnitine on lipid metabolism of sheep. Biol Trace Elem Res 155(2):221–227. https://doi.org/10.1007/s12011-013-9790-9
Saltiel AR, Kahn CR (2001) Insulin signalling and the regulation of glucose and lipid metabolism. Nature 414(6865):799–806. https://doi.org/10.1038/414799a
Xu X, Liu L, Long SF, Piao XS, Ward TL, Ji F (2017) Effects of chromium methionine supplementation with different sources of zinc on growth performance, carcass traits, meat quality, serum metabolites, endocrine parameters, and the antioxidant status in growing-finishing pigs. Biol Trace Elem Res 179(1):70–78. https://doi.org/10.1007/s12011-017-0935-0
Tian YY, Zhang LY, Dong B, Cao J, Xue JX, Gong LM (2014) Effects of chromium methionine supplementation on growth performance, serum metabolites, endocrine parameters, antioxidant status, and immune traits in growing pigs. Biol Trace Elem Res 162(1-3):134–141. https://doi.org/10.1007/s12011-014-0147-9
Jamilian M, Bahmani F, Siavashani MA, Mazloomi M, Asemi Z, Esmaillzadeh A (2016) The effects of chromium supplementation on endocrine profiles, biomarkers of inflammation, and oxidative stress in women with polycystic ovary syndrome: a randomized, double-blind, placebo-controlled trial. Biol Trace Elem Res 172(1):72–78. https://doi.org/10.1007/s12011-015-0570-6
Tan GY, Zheng SS, Zhang MH, Feng JH, Xie P, Bi JM (2008) Study of oxidative damage in growing-finishing pigs with continuous excess dietary chromium picolinate intake. Biol Trace Elem Res 126(1-3):129–140. https://doi.org/10.1007/s12011-008-8207-7
Zer A, Bakacak M, Kiran H, Ercan O, Kostu B, Kanat-Pektas M, Kilinc M, Aslan F (2016) Increased oxidative stress is associated with insulin resistance and infertility in polycystic ovary syndrome. Ginekol Pol 87(11):733–738. https://doi.org/10.5603/GP.2016.0079
Attaran M, Pasqualotto E, Falcone T, Goldberg JM, Miller KF, Agarwal A, Sharma RK (2000) The effect of follicular fluid reactive oxygen species on the outcome of in vitro fertilization. Int J Fertil Womens Med 45(5):314–320
Dimmeler S, Haendeler J, Sause A, Zeiher AM (1998) Nitric oxide inhibits APO-1/Fas-mediated cell death. Cell Growth Differ 9(5):415–422
Tamarit J, Cabiscol E, Ros J (1998) Identification of the major oxidatively damaged proteins in Escherichia coli cells exposed to oxidative stress. J Biol Chem 273(5):3027–3032. https://doi.org/10.1074/jbc.273.5.3027
Pekel A, Gonenc A, Turhan NO, Kafali H (2015) Changes of sFas and sFasL, oxidative stress markers in serum and follicular fluid of patients undergoing IVF. J Assist Reprod Genet 32(2):233–241. https://doi.org/10.1007/s10815-014-0396-8
Jain SK, Kannan K (2001) Chromium chloride inhibits oxidative stress and TNF-alpha secretion caused by exposure to high glucose in cultured U937 monocytes. Biochem Biophys Res Commun 289(3):687–691. https://doi.org/10.1006/bbrc.2001.6026
Jain SK, Patel P, Rogier K (2006) Trivalent chromium inhibits protein glycosylation and lipid peroxidation in high glucose-treated erythrocytes. Antioxid Redox Signal 8(1-2):238–241. https://doi.org/10.1089/ars.2006.8.238
Funding
This study was supported by a grant from the Vice-chancellor for Research, Arak University of Medical Sciences, Arak, Iran.
Author information
Authors and Affiliations
Contributions
ZA helped inthe conception, design, and statistical analysis of the manuscript. MJ, SZ, MA-S, MK, AM, and VO contributed in data collection and manuscript drafting. ZA supervised the study.
Corresponding author
Ethics declarations
Conflicts of Interest
The authors declare that they have no conflict of interest.
Additional information
Clinical trial registration number
http://www.irct.ir: IRCT201706075623N120.
Rights and permissions
About this article
Cite this article
Jamilian, M., Zadeh Modarres, S., Amiri Siavashani, M. et al. The Influences of Chromium Supplementation on Glycemic Control, Markers of Cardio-Metabolic Risk, and Oxidative Stress in Infertile Polycystic ovary Syndrome Women Candidate for In vitro Fertilization: a Randomized, Double-Blind, Placebo-Controlled Trial. Biol Trace Elem Res 185, 48–55 (2018). https://doi.org/10.1007/s12011-017-1236-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-017-1236-3