Abstract
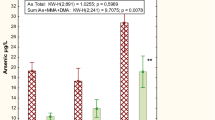
Inorganic arsenic (iAs) is a carcinogen and could increase the risks of bladder, lung, and skin cancer. Mining and smelting of non-ferrous metals are common occupational arsenic exposures. In this study, 125 individuals working in non-ferrous metal smelting plants were separated into two groups according to urinary total arsenic (TAs) levels: group 1, TAs < 100 μg/g Cr; group 2, TAs ≥ 100 μg/g Cr. Demographic characteristics of participants were obtained by questionnaire interview. Levels of E-cadherin soluble ectodomain fragment (sEcad) and epidermal growth factor (EGF) in workers urine were determined by ELISA test. We found that concentrations of sEcad and EGF present in urine were significantly elevated in the high urinary arsenic group 2 compared with the low urinary arsenic group 1. Urinary levels of the shedding of E-cadherin soluble ectodomain fragment (sEcad) and epidermal growth factor (EGF) were positively related to the concentrations of iAs in urine after adjusting for the confounding effects. A positive correlation between sEcad and EGF concentrations in urine was also observed. In order to verify the effects of iAs on sEcad and EGF, the human uroepithelial cell line (SV-HUC-1) was treated with NaAsO2 for 24 h in vitro. sEcad and EGF levels in the 4 μM NaAsO2-treated SV-HUC-1 cell medium significantly increased compared to the control group. In conclusion, urinary levels of sEcad and EGF increased in higher urinary arsenic workers of non-ferrous metal plants and are closely associated with urinary iAs concentration. The results suggested that sEcad and EGF may potentially be preclinical prognostic factors of bladder injury and early detection in arsenic exposure individuals.



Similar content being viewed by others
References
Charles E, Thomas DS, Dewey D, Davey M, Ngallaba SE, Konje E (2013) A cross-sectional survey on knowledge and perceptions of health risks associated with arsenic and mercury contamination from artisanal gold mining in Tanzania. BMC Public Health 13:74
Wang QQ, Thomas DJ, Naranmandura H (2015) Importance of being thiomethylated: formation, fate, and effects of methylated thioarsenicals. Chem Res Toxicol 28:281–289
Mohammed Abdul KS, Jayasinghe SS, Chandana EP, Chandana EP, Jayasumana C, De Silva PM (2015) Arsenic and human health effects: a review. Environ Toxicol Pharmacol 40:828–846
Marchiset-Ferlay N, Savanovitch C, Sauvant-Rochat MP (2012) What is the best biomarker to assess arsenic exposure via drinking water? Environ Int 39:150–171
IARC (International Agency for Research on Cancer) (2012) A review of human carcinogens: arsenic, metals, fibres, and dusts. Vol. 100c,
Chiou HY, Chiou ST, Hsu YH, Chou YL, Tseng CH, Wei ML, Chen CJ (2001) Incidence of transitional cell carcinoma and arsenic in drinking water: a follow-up study of 8,102 residents in an arseniasis-endemic area in northeastern Taiwan. Am J Epidemiol 153:411–418
Smith AH, Goycolea M, Haque R, Biggs ML (1998) Marked increase in bladder and lung cancer mortality in a region of Northern Chile due to arsenic in drinking water. Am J Epidemiol 147:660–669
Steinmaus C, Ferreccio C, Acevedo J, Yuan Y, Liaw J, Durán V et al (2014) Increased lung and bladder cancer incidence in adults after in utero and early-life arsenic exposure. Cancer Epidemiol Biomark Prev 23:1529–1538
Steinmaus CM, Ferreccio C, Romo JA, Yuan Y, Cortes S, Marshall G et al (2013) Drinking water arsenic in northern Chile: high cancer risks 40 years after exposure cessation. Cancer Epidemiol Biomark Prev 22:623–630
Semb H, Christofori G (1998) The tumor-suppressor function of E-cadherin. Am J Hum Genet 63:1588–1593
Maretzky T, Reiss K, Ludwig A, Buchholz J, Scholz F, Proksch E et al (2005) ADAM10 mediates E-cadherin shedding and regulates epithelial cell–cell adhesion, migration, and beta-catenin translocation. Proc Natl Acad Sci U S A 102:9182–9187
Brouxhon SM, Kyrkanides S, Teng X, Athar M, Ghazizadeh S, Simon M et al (2014) Soluble E-cadherin: a critical oncogene modulating receptor tyrosine kinases, MAPK and PI3K/Akt/mTOR signaling. Oncogene 33:225–235
Charalabopoulos K, Gogali A, Dalavaga Y, Daskalopoulos G, Vassiliou M, Bablekos G et al (2006) The clinical significance of soluble E-cadherin in nonsmall cell lung cancer. Exp Oncol 28:83–85
Salama RH, Selem TH, El-Gammal M, Elhagagy AE, Bakar SM (2013) Urinary tumor markers could predict survival in bladder carcinoma. Indian J Clin Biochem 28:265–271
Shi B, Laudon V, Yu S, Dong D, Zhu Y, Xu Z (2008) E-cadherin tissue expression and urinary soluble forms of E-cadherin in patients with bladder transitional cell carcinoma. Urol Int 81:320–324
Brouxhon SM, Kyrkanides S, Teng X, O’Banion MK, Clarke R, Byers S et al (2014) Soluble-E-cadherin activates HER and IAP family members in HER2+ and TNBC human breast cancers. Mol Carcinog 53:893–906
Najy AJ, Day KC, Day ML (2008) The ectodomain shedding of E-cadherin by ADAM15 supports ErbB receptor activation. J Biol Chem 283:18393–18401
Inge LJ, Barwe SP, D’Ambrosio J, Gopal J, Lu K, Ryazantsev S et al (2011) Soluble E-cadherin promotes cell survival by activating epidermal growth factor receptor. Exp Cell Res 317:838–848
Kari C, Chan TO, Rocha de Quadros M, Rodeck U (2003) Targeting the epidermal growth factor receptor in cancer: apoptosis takes center stage. Cancer Res 63:1–5
Eblin KE, Bredfeldt TG, Buffington S, Gandolfi AJ (2007) Mitogenic signal transduction caused by monomethylarsonous acid in human bladder cells: role in arsenic-induced carcinogenesis. Toxicol Sci 95:321–330
Simeonova PP, Wang S, Hulderman T, Luster MI (2002) c-Src dependent activation of the epidermal growth factor receptor and mitogenactivated protein kinase pathway by arsenic. Role in carcinogenesis. J Biol Chem 277:2945–2950
Grivas PD, Day M, Hussain M (2011) Urothelial carcinomas: a focus on human epidermal receptors signaling. Am J Transl Res 3:362–373
Cheng JC, Klausen C, Leung PC (2010) Hydrogen peroxide mediates EGF-induced down-regulation of E-cadherin expression via p38 MAPK and snail in human ovarian cancer cells. Mol Endocrinol 24:1569–1580
Cheng JC, Qiu X, Chang HM, Leung PC (2013) HER2 mediates epidermal growth factor-induced down-regulation of E-cadherin in human ovarian cancer cells. Biochem Biophys Res Commun 434:81–86
Hu QP, Kuang JY, Yang QK, Bian XW, Yu SC (2016) Beyond a tumor suppressor: soluble E-cadherin promotes the progression of cancer. Int J Cancer 138:2804–2812
Brouxhon SM, Kyrkanides S, Raja V, Silberfeld A, Teng X, Trochesset D et al (2014) Ectodomain-specific E-cadherin antibody suppresses skin SCC growth and reduces tumor grade: a multitargeted therapy modulating RTKs and the PTEN–p53–MDM2 Axis. Mol Cancer Ther 13:1791–1802
De Wever O, Derycke L, Hendrix A, De Meerleer G, Godeau F, Depypere H et al (2007) Soluble cadherins as cancer biomarkers. Clin Exp Metastasis 24:685–697
Sewpaul A, French JJ, Khoo TK, Kernohan M, Kirby JA, Charnley RM (2009) Soluble E-cadherin: an early marker of severity in acute pancreatitis. HPB Surg 397375
Liu S, Sun Q, Wang F, Zhang L, Song Y, Xi S et al (2014) Arsenic induced overexpression of inflammatory cytokines based on the human urothelial cell model in vitro and urinary secretion of individuals chronically exposed to arsenic. Chem Res Toxicol 27:1934–1942
Sun G, Xu Y, Li X, Jin Y, Li B, Sun X (2007) Urinary arsenic metabolites in children and adults exposed to arsenic in drinking water in Inner Mongolia, China. Environ Health Perspect 115:648–652
Hayashido Y, Hamana T, Yoshioka Y, Kitano H, Koizumi K, Okamoto T (2005) Plasminogen activator/plasmin system suppresses cell-cell adhesion of oral squamous cell carcinoma cells via proteolysis of E-cadherin. Int J Oncol 27:693–698
Shariat SF, Matsumoto K, Casella R, Jian W, Lerner SP (2005) Urinary levels of soluble E-cadherin in the detection of transitional cell carcinoma of the urinary bladder. Eur Urol 48:69–76
Symowicz J, Adley BP, Gleason KJ, Johnson JJ, Ghosh S, Fishman DA et al (2007) Engagement of collagen-binding integrins promotes matrix metalloproteinase-9-dependent E-cadherin ectodomain shedding in ovarian carcinoma cells. Cancer Res 67:2030–2039
Achanzar WE, Moyer CF, Marthaler LT, Gullo R, Chen SJ, French MH et al (2007) Urine acidification has no effect on peroxisome proliferator-activated receptor (PPAR) signaling or epidermal growth factor (EGF) expression in rat urinary bladder urothelium. Toxicol Appl Pharmacol 223:246–256
Izumi K, Zheng Y, Li Y, Zaengle J, Miyamoto H (2012) Epidermal growth factor induces bladder cancer cell proliferation through activation of the androgen receptor. Int J Oncol 41:1587–1592
Grabowska MM, Sandhu B, Day ML (2012) EGF promotes the shedding of soluble E-cadherin in an ADAM10-dependent manner in prostate epithelial cells. Cell Signal 24:532–538
Melak D, Ferreccio C, Kalman D, Parra R, Acevedo J, Pérez L et al (2014) Arsenic methylation and lung and bladder cancer in a case control study in northern Chile. Toxicol Appl Pharmacol 274:225–231
Acknowledgements
This work was supported by the National Natural Science Foundation of China (NSFC) (81373023 and 81673207) and the Program for Liaoning Innovative Research Team in University (LT2015028).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
All participants provided informed consents before the questionnaire interview and then donated urine. The study was approved by the China Medical University Ethics Committee.
Conflict of Interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Liu, J., Jin, P., Liu, S. et al. sEcad and EGF Levels Increased in Urine of Non-ferrous Metal Workers and Medium of Uroepithelial Cell Line Treated by Arsenic. Biol Trace Elem Res 183, 32–39 (2018). https://doi.org/10.1007/s12011-017-1124-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-017-1124-x