Abstract
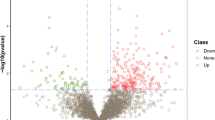
Keshan disease (KD) is an endemic cardiomyopathy with high mortality. Selenium (Se) and zinc (Zn) deficiencies are closely related to KD. The molecular mechanism of KD pathogenesis is still unclear. There are only few studies on the interaction of trace elements and proteins associated with the pathogenesis of KD. In this study, isobaric tags for relative and absolute quantitation (iTRAQ)-coupled two-dimensional liquid chromatography tandem mass spectrometry (2DLC-MS/MS) technique analysis was used to analyze the differential expression of proteins from serum samples. Comparative Toxicogenomics Database (CTD) was used to screen Se- and Zn-associated proteins. Then, pathway and network analyses of Se- and Zn-associated proteins were constituted by Cytoscape ClueGO and GeneMANIA plugins. One hundred and five differentially expressed proteins were obtained by 2DLC-MS/MS, among them 19 Se- and 3 Zn-associated proteins. Fifty-two pathways were identified from ClueGO and 1 network from GeneMANIA analyses. The results showed that Se-associated proteins STAT3 and MAPK1 and Zn-associated proteins HIF1A and PARP1, the proteins involved in HIF-1 signaling pathway and apoptosis pathway, may play significant roles in the pathogenesis of KD. The approach of this study would be also beneficial for further dissecting molecular mechanism of other trace element-associated disease.



Similar content being viewed by others
References
Zhu Y, Lai B, Niu X, Wei J, Tan W, Wang X (2014) Long-term prognostic value of major and minor ECG abnormalities in latent Keshan disease with suspect chronic Keshan disease. J Epidemiol 24:385–391. doi:10.2188/jea.JE20130180
Lei C, Niu X, Ma X, Wei J (2011) Is selenium deficiency really the cause of Keshan disease? Environ Geochem Health 33:183–188. doi:10.1007/s10653-010-9331-9
Blankenberg S, Rupprecht HJ, Bickel C, Torzewski M, Hafner G, Tiret L, Smieja M, Cambien F, Meyer J, Lackner KJ, AtheroGene Investigators (2003) Glutathione peroxidase 1 activity and cardiovascular events in patients with coronary artery disease. N Engl J Med 349:1605–1613. doi:10.1056/NEJMoa030535
Chen J (2012) An original discovery: selenium deficiency and Keshan disease (an endemic heart disease). Asia Pac J Clin Nutr 21:320–326
Hongyan Z, Nan G, Jie J, Dan Y (2016) Five kinds of trace elements in keshan disease comparison with levels in the blood of the patients with dilated cardiomyopathy. Chin J Control Endem Dis 31:404–406
He S, Tan W, Wang S, Wu C, Wang P, Wang B, Su X, Zhao J, Guo X, Xiang Y (2014) Genome-wide study reveals an important role of spontaneous autoimmunity, cardiomyocyte differentiation defect and anti-angiogenic activities in gender-specific gene expression in Keshan disease. Chin Med J 127:72–78
Wang B, He S, Lei Y, Tan W, Zhang F, Wang P, Zhu Y, Guo X (2014) Microarray-based gene expression profiles related to Keshan disease. J Xi'an Jiaotong Univ (Med Sci) 35:163–168
He S, Tan W, Wang J, Wang P, Xiang Y (2013) Screening of differentially expressed proteins in serum from subjects with Keshan disease by two-dimensional electrophoresis and mass and mass spectrometry. J Hygiene Res 42:424–428
Alvarez S, Berla BM, Sheffield J, Cahoon RE, Jez JM, Hicks LM (2009) Comprehensive analysis of the Brassica juncea root proteome in response to cadmium exposure by complementary proteomic approaches. Proteomics 9:2419–2431. doi:10.1002/pmic.200800478
Davis AP, Grondin CJ, Johnson RJ, Sciaky D, King BL, McMorran R, Wiegers J, Wiegers TC, Mattingly CJ (2017) The Comparative Toxicogenomics Database: update 2017. Nucleic Acids Res 45:D972–D978. doi:10.1093/nar/gkw838
Mlecnik B, Galon J, Bindea G (2017) Comprehensive functional analysis of large lists of genes and proteins. J Proteomics. doi:10.1016/j.jprot.2017.03.016
Sabaouni I, Vannier B, Moussa A, Ibrahimi A (2016) Microarray integrated analysis of a gene network for the CD36 myocardial phenotype. Bioinformation 12:332–339. doi:10.6026/97320630012332
Shan H, Yan R, Diao J, Lin L, Wang S, Zhang M, Zhang R, Wei J (2015) Involvement of caspases and their upstream regulators in in vitro apoptosis in a rat model of selenium deficiency-induced dilated cardiomyopathy. J Trace Elem Med Biol 31:85–89. doi:10.1016/j.jtemb.2015.03.005
Metin G, Atukeren P, Alturfan AA, Gulyasar T, Kaya M, Gumustas MK (2003) Lipid peroxidation, erythrocyte superoxide-dismutase activity and trace metals in young male footballers. Yonsei Med J 44:979–986. doi:10.3349/ymj.2003.44.6.979
Jang Y, Wang H, Xi J, Mueller RA, Norfleet EA, Xu Z (2007) NO mobilizes intracellular Zn2+ via cGMP/PKG signaling pathway and prevents mitochondrial oxidant damage in cardiomyocytes. Cardiovasc Res 75:426–433. doi:10.1016/j.cardiores.2007.05.015
Cesselli D, Jakoniuk I, Barlucchi L, Beltrami AP, Hintze TH, Nadal-Ginard B, Kajstura J, Leri A, Anversa P (2001) Oxidative stress-mediated cardiac cell death is a major determinant of ventricular dysfunction and failure in dog dilated cardiomyopathy. Circ Res 89:279–286. doi:10.1161/hh1501.094115
Pei J, Fu W, Yang L, Zhang Z, Liu Y (2013) Oxidative stress is involved in the pathogenesis of Keshan disease (an endemic dilated cardiomyopathy) in China. Oxidative Med Cell Longev 2013:474203. doi:10.1155/2013/474203
Zhang M, Wei J, Pan X, Shan H, Yan R, Xue J, Zhu Y, Lin L (2013) Change of cardiac mitochondrial STAT3 activity in rats with seleniumdeficiency and its relation with myocardial injury. J South Med Univ 33:967–971
Stapleton SR, Garlock GL, Foellmi-Adams L, Kletzien RF (1997) Selenium: potent stimulator of tyrosyl phosphorylation and activator of MAP kinase. Biochim Biophys Acta 1355:259–269. doi:10.1016/S0167-4889(96)00140-1
Zhang X, Liang D, Fan J, Lian X, Zhao Y, Wang X, Chi ZH, Zhang P (2016) Zinc attenuates tubulointerstitial fibrosis in diabetic nephropathy via inhibition of HIF through PI-3K signaling. Biol Trace Elem Res 173:372–383. doi:10.1007/s12001-016-0661-z
Cooper KL, Dashner EJ, Tsosie R, Cho YM, Lewis J, Hudson LG (2016) Inhibition of poly(ADP-ribose)polymerase-1 and DNA repair by uranium. Toxicol Appl Pharmacol 291:13–20. doi:10.1016/j.taap.2015.11.017
Fuyu Y (2006) Keshan disease and mitochondrial cardiomyopathy. Sci China C Life Sci 49:513–518
Diao J, Wei J, Yan R, Liu X, Li Q, Lin L, Zhu Y, Li H (2016) Rosmarinic acid suppressed high glucose-induced apoptosis in H9c2 cells by ameliorating the mitochondrial function and activating STAT3. Biochem Biophys Res Commun 477:1024–1030. doi:10.1016/j.bbrc.2016.07.024
He SL, Tan WH, Zhang ZT, Zhang F, Qu CJ, Lei YX, Zhu YH, Yu HJ, Xiang YZ, Guo X (2013) Mitochondrial-related gene expression profiles suggest an important role of PGC-1alpha in the compensatory mechanism of endemic dilated cardiomyopathy. Exp Cell Res 319:2604–2616. doi:10.1016/j.yexcr.2013.07.018
Zhao J, Li L, Peng L (2015) MAPK1 up-regulates the expression of MALAT1 to promote the proliferation of cardiomyocytes through PI3K/AKT signaling pathway. Int J Clin Exp Pathol 8:15947–15953
Nanayakkara G, Alasmari A, Mouli S, Eldoumani H, Quindry J, McGinnis G, Fu X, Berlin A, Peters B, Zhong J, Amin R (2015) Cardioprotective HIF-1alpha-frataxin signaling against ischemia-reperfusion injury. Am J Physiol Heart Circ Physiol 309:H867–H879. doi:10.1152/ajpheart.00875.2014
Gero D, Szabo C (2015) Salvage of nicotinamide adenine dinucleotide plays a critical role in the bioenergetic recovery of post-hypoxic cardiomyocytes. Br J Pharmacol 172:4817–4832. doi:10.1111/bph.13252
Acknowledgments
This study was supported by the National Natural Science Foundation of China (grant numbers 81273008 and 81402638).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of Interest
The authors declare that they have no conflicts of interest.
Electronic supplementary material
Table S1
(DOCX 24 kb)
Rights and permissions
About this article
Cite this article
Wang, S., Lv, Y., Wang, Y. et al. Network Analysis of Se-and Zn-related Proteins in the Serum Proteomics Expression Profile of the Endemic Dilated Cardiomyopathy Keshan Disease. Biol Trace Elem Res 183, 40–48 (2018). https://doi.org/10.1007/s12011-017-1063-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-017-1063-6