Abstract
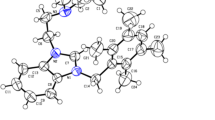
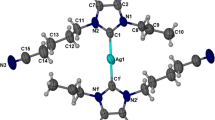
Synthesis and anticancer studies of three symmetrically and non-symmetrically substituted silver(I)-N-Heterocyclic carbene complexes of type [(NHC)2-Ag]PF6 (7–9) and their respective (ligands) benzimidazolium salts (4–6) are described herein. Compound 5 and Ag-NHC-complex 7 were characterized by the single crystal X-ray diffraction technique. Structural studies for 7 showed that the silver(I) center has linear C-Ag-C coordination geometry (180.00(10)o). Other azolium and Ag-NHC analogues were confirmed by H1 and C13-NMR spectroscopy. The synthesized analogues were biologically characterized for in vitro anticancer activity against three cancer cell lines including human colorectal cancer (HCT 116), breast cancer (MCF-7), and erythromyeloblastoid leukemia (K-562) cell lines and in terms of in vivo acute oral toxicity (IAOT) in view of agility and body weight of female rats. In vitro anticancer activity showed the values of IC50 in range 0.31–17.9 μM in case of K-562 and HCT-116 cancer cell lines and 15.1–35.2 μM in case of MCF-7 while taking commercially known anticancer agents 5-fluorouracil, tamoxifen, and betulinic acid which have IC50 values 5.2, 5.5, and 17.0 μM, respectively. In vivo study revealed vigor and agility of all test animals which explores the biocompatibility and non-toxicity of the test analogues.









Similar content being viewed by others
References
Gardiner, M. G., & Ho, C. C. (2018). Recent advances in bidentate bis (N-heterocyclic carbene) transition metal complexes and their applications in metal-mediated reactions. Coordination Chemistry Reviews, 375, 373–388.
El-Kady, D. S., Abd Rabou, A. A., Tantawy, M. A., Abdel-Rahman, A. A.-H., Abdel-Megeed, A. A.-S., AbdElhalim, M. M., & Elmegeed, G. A. (2019). Synthesis and evaluation of novel cholestanoheterocyclic steroids as anticancer agents. Applied Biochemistry and Biotechnology, 188(3), 635–662. https://doi.org/10.1007/s12010-018-02943-6.
Kaloğlu, N., Kaloğlu, M., Özdemir, İ., Günal, S., & Özdemir, İ. (2017). Silver–N-heterocyclic carbene complexes: synthesis, characterization, and antimicrobial properties. Journal of the Chinese Chemical Society, 64(4), 420–426.
Xu, L., Hong, M., Wang, Y., Li, M., Li, H., Nair, M. P. N., & Li, C.-Z. (2016). Tunable synthesis solid or hollow Au–Ag nanostructure, assembled with GO and comparative study of their catalytic properties. Science Bulletin, 61(19), 1525–1535. https://doi.org/10.1007/s11434-016-1165-0.
Hopkinson MN., Richter C., Schedler M., Glorius F. (2014). An overview of N-heterocyclic carbenes. Nature 510(7506):485.
Meng, G., Kakalis, L., Nolan, S. P., & Szostak, M. (2019). A simple 1H NMR method for determining the σ-donor properties of N-heterocyclic carbenes. Tetrahedron Letters, 60(4), 378–381.
Munz, D. (2018). Pushing electrons▪ which carbene ligand for which application? Organometallics, 37(3), 275–289.
Wang, M. H., & Scheidt, K. A. (2016). Cooperative catalysis and activation with N-heterocyclic carbenes. Angewandte Chemie International Edition, 55(48), 14912–14922.
Pudlarz, A. M., Ranoszek-Soliwoda, K., Czechowska, E., Tomaszewska, E., Celichowski, G., Grobelny, J., & Szemraj, J. (2019). A study of the activity of recombinant Mn-superoxide dismutase in the presence of gold and silver nanoparticles. Applied Biochemistry and Biotechnology, 187(4), 1551–1568. https://doi.org/10.1007/s12010-018-2896-y.
Pudlarz, A. M., Czechowska, E., Ranoszek-Soliwoda, K., Tomaszewska, E., Celichowski, G., Grobelny, J., & Szemraj, J. (2018). Immobilization of recombinant human catalase on gold and silver nanoparticles. Applied Biochemistry and Biotechnology, 185(3), 717–735. https://doi.org/10.1007/s12010-017-2682-2.
Mercs L., & Albrecht M. (2010). Beyond catalysis: N-heterocyclic carbene complexes as components for medicinal, luminescent, and functional materials applications. Chemical Society Reviews. 39(6):1903–12.
Medici, S., Peana, M., Crisponi, G., Nurchi, V. M., Lachowicz, J. I., Remelli, M., & Zoroddu, M. A. (2016). Silver coordination compounds: a new horizon in medicine. Coordination Chemistry Reviews, 327-328, 349–359. https://doi.org/10.1016/j.ccr.2016.05.015.
Soleymani-Babadi, S., Beheshti, A., Bahrani-Pour, M., Mayer, P., Motamedi, H., Trzybiński, D., & Wozniak, K. (2019). Synthesis, structural characterization, photophysical properties, and antibacterial assessment of silver(I)-thione coordination polymers based on a competition between nitrate anion and coanions CF3SO3–, ClO4–, BF4–, PF6–, and SbF6. Crystal Growth & Design, 19(9), 4934–4948. https://doi.org/10.1021/acs.cgd.9b00038.
Habib, A., Iqbal, M. A., Bhatti, H. N., & Shahid, M. (2019). Effect of ring substitution on synthesis of benzimidazolium salts and their silver(I) complexes: characterization, electrochemical studies and evaluation of anticancer potential. Transition Metal Chemistry. https://doi.org/10.1007/s11243-019-00321-7.
Habib, A., Iqbal, M. A., & Bhatti, H. N. (2019). Polynuclear Ag(I)-N-heterocyclic carbene complexes: Synthesis, electrochemical and in vitro anticancer study against human breast cancer and colon cancer. Journal of Coordination Chemistry, 1–15. https://doi.org/10.1080/00958972.2019.1632837.
Habib, A., MN, V., Iqbal, M. A., Bhatti, H. N., MBK, A., & AMSA, M. (2019). Unsymmetrically substituted benzimidazolium based silver(I)-N-heterocyclic carbene complexes: Synthesis, characterization and in vitro anticancer study against human breast cancer and colon cancer. Journal of Saudi Chemical Society. https://doi.org/10.1016/j.jscs.2019.03.002.
Noor u, H., Islam, S., Zia, M., William, K., Abbas Fakhar, I., Umar Muhammad, I., Iqbal Muhammad, A., & Mannan, A. (2018). Anticancer, antimicrobial and antioxidant potential of sterically tuned bis-N-heterocyclic salts. Zeitschrift für Naturforschung C, 74. https://doi.org/10.1515/znc-2018-0095.
Habib, A., Iqbal, M. A., & Bhatti, H. N. (2019). Polynuclear Ag (I)-N-heterocyclic carbene complexes: Synthesis, electrochemical and in vitro anticancer study against human breast cancer and colon cancer. Journal of Coordination Chemistry, 1–15.
Varna, D., Zainuddin, D. I., Hatzidimitriou, A. G., Psomas, G., Pantazaki, A. A., Papi, R., Angaridis, P., & Aslanidis, P. (2019). Homoleptic and heteroleptic silver(I) complexes bearing diphosphane and thioamide ligands: Synthesis, structures, DNA interactions and antibacterial activity studies. Materials Science and Engineering: C, 99, 450–459.
Iqbal, M. A., Haque, R. A., Ahamed, M. B. K., Majid, A. A., & Al-Rawi, S. S. (2013). Synthesis and anticancer activity of para-xylyl linked bis-benzimidazolium salts and respective Ag (I) N-heterocyclic carbene complexes. Medicinal Chemistry Research, 22(5), 2455–2466.
Haque, R. A., Iqbal, M. A., Budagumpi, S., Khadeer Ahamed, M. B., Abdul Majid, A. M., & Hasanudin, N. (2013). Binuclear meta-xylyl-linked Ag (I)-N-heterocyclic carbene complexes of N-alkyl/aryl-alkyl-substituted bis-benzimidazolium salts: Synthesis, crystal structures and in vitro anticancer studies. Applied Organometallic Chemistry, 27(4), 214–223.
Asif, M., Iqbal, M. A., Hussein, M. A., Oon, C. E., Haque, R. A., Ahamed, M. B. K., Majid, A. S. A., & Majid, A. M. S. A. (2016). Human colon cancer targeted pro-apoptotic, anti-metastatic and cytostatic effects of binuclear silver(I)–N-heterocyclic carbene (NHC) complexes. European Journal of Medicinal Chemistry, 108, 177–187.
Fatima, T., Haque, R. A., Razali, M. R., Ahmad, A., Iqbal, M. A., Asif, M., Ahamed, M. B. K., & Abdul Majid, A. M. S. (2016). Synthesis, crystal structure, in vitro anticancer and in vivo acute oral toxicity studies of tetramethylene linked bis-benzimidazolium salts and their respective dinuclear Ag(I)–NHC complexes. Journal of Coordination Chemistry, 69(22), 3367–3383. https://doi.org/10.1080/00958972.2016.1230670.
Haque, R. A., Hasanudin, N., Iqbal, M. A., Ahmad, A., Hashim, S., Abdul Majid, A., & Ahamed, M. B. K. (2013). Synthesis, crystal structures, in vitro anticancer, and in vivo acute oral toxicity studies of bis-imidazolium/benzimidazolium salts and respective dinuclear Ag(I)-N-heterocyclic carbene complexes. Journal of Coordination Chemistry, 66(18), 3211–3228. https://doi.org/10.1080/00958972.2013.831406.
Liu, Q.-X., Yin, L.-N., & Feng, J.-C. (2007). New N-heterocyclic carbene silver (I) and mercury (II) 2-D supramolecular layers by the π–π stacking interactions. Journal of Organometallic Chemistry, 692(17), 3655–3663.
Liu, Q.-X., Zhao, X.-J., Wu, X.-M., Guo, J.-H., & Wang, X.-G. (2007). New mercury (II) and silver (I) complexes containing NHC metallacrown ethers with the π–π stacking interactions. Journal of Organometallic Chemistry, 692(25), 5671–5679.
Baker, M. V., Brown, D. H., Haque, R. A., Skelton, B. W., & White, A. H. (2004). Dinuclear N-heterocyclic carbene complexes of silver (I), derived from imidazolium-linked cyclophanes. Dalton Transactions, 21, 3756–3764.
Hahn, F. E., Radloff, C., Pape, T., & Hepp, A. (2008). Synthesis of silver(I) and gold(I) complexes with cyclic tetra-and hexacarbene ligands. Chemistry–A European Journal, 14(35), 10900–10904.
Liu, Q.-X., Yu, J., Zhao, X.-J., Liu, S.-W., Yang, X.-Q., Li, K.-Y., & Wang, X.-G. (2011). Mercury (II), copper (II) and silver (I) complexes with ether or diether functionalized bis-NHC ligands: Synthesis and structural studies. CrystEngComm, 13(12), 4086–4096.
Kankala, S., Thota, N., Björkling, F., Taylor, M. K., Vadde, R., & Balusu, R. (2019). Silver carbene complexes: An emerging class of anticancer agents. Drug Development Research, 80(2), 188–199.
Iqbal, M. A., Haque, R. A., Budagumpi, S., Ahamed, M. B. K., & Majid, A. M. A. (2013). Short metal–metal separations and in vitro anticancer studies of a new dinuclear silver (I)-N-heterocyclic carbene complex of para-xylyl-linked bis-benzimidazolium salt. Inorganic Chemistry Communications, 28, 64–69.
Teyssot, M. L., Jarrousse, A. S., Chevry, A., De Haze, A., Beaudoin, C., Manin, M., Nolan, S. P., Díez-González, S., Morel, L., & Gautier, A. (2009). Toxicity of copper (I)–NHC complexes against human tumor cells: Induction of cell cycle arrest, apoptosis, and DNA cleavage. Chemistry–A European Journal, 15(2), 314–318.
Haque, R. A., Hasanudin, N., Iqbal, M. A., Ahmad, A., Hashim, S., Abdul Majid, A., & Ahamed, M. B. K. (2013). Synthesis, crystal structures, in vitro anticancer, and in vivo acute oral toxicity studies of bis-imidazolium/benzimidazolium salts and respective dinuclear Ag (I)-N-heterocyclic carbene complexes. Journal of Coordination Chemistry, 66(18), 3211–3228.
Gautier, A., & Cisnetti, F. (2012). Advances in metal–carbene complexes as potent anti-cancer agents. Metallomics, 4(1), 23–32.
Walum, E. (1998). Acute oral toxicity. Environmental Health Perspectives, 106(suppl 2), 497–503.
Acknowledgments
The authors are thankful to Higher Education Commission (HEC) of Pakistan for providing grant National Research Programme for Universities No. NRPU-8396 & NRPU-8198. They are also very much obliged to Rosenani A. Haque, School of Chemical Sciences as well as to Mohamed B. Khadeer Ahamed and Amin M. S. Abdul Majid of EMAN Research and Testing Laboratory, School of Pharmaceutical Sciences of Universiti Sains Malaysia, 11800 USM, Penang, Malaysia for their technical support and proof-reading of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Appendix A Supplementary Material
Appendix A Supplementary Material
CCDC 916242 and 916239 contain the supplementary crystallographic data. The supplementary crystallographic data for salt 5 and carbene complex 7 is available free of charge at CCDC 916242 and 916239 of Cambridge Crystallographic Data Centre, 12 Union Road, Cambridge CB2 1EZ,UK; fax (+44) 1223–336-033 or e-mail deposit@ccdc.cam.ac.uk or using link http://www.ccdc.cam.ac.uk/conts/retrieving.html. Moreover, the effects of increasing amounts of test compound on the percentage inhibition of cell proliferation are also available.
Rights and permissions
About this article
Cite this article
Atif, M., Bhatti, H.N., Haque, R.A. et al. Synthesis, Structure, and Anticancer Activity of Symmetrical and Non-symmetrical Silver(I)-N-Heterocyclic Carbene Complexes. Appl Biochem Biotechnol 191, 1171–1189 (2020). https://doi.org/10.1007/s12010-019-03186-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-019-03186-9