Abstract
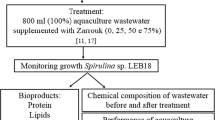
The composition of brackish groundwater from Brazilian backlands contains important elements necessary for metabolism in microalgae. This study evaluated the use of 100% brackish groundwater with different amounts of Zarrouk nutrients for Spirulina sp. LEB 18 cultivation. The growth parameters and biomass composition, including the concentrations of proteins, carbohydrates, ash, lipids, and fatty acids, were evaluated. The best growth parameter results were obtained in the assay using 100% brackish groundwater and only 25% of Zarrouk nutrients, which were equal to those obtained for the control culture. The concentrations of carbohydrates and polyunsaturated fatty acids were increased by as much as 4- and 3.3-fold, respectively, when brackish groundwater was used in the cultures. The lipid profile demonstrated that the biomass had the potential for use in biodiesel production. The use of brackish groundwater is a sustainable, economical way to obtain high-quality biomass for different applications during Spirulina sp. LEB 18 cultivation.


Similar content being viewed by others
References
McNeill, K., Macdonald, K., Singh, A., & Binns, A. D. (2017). Food and water security: Analysis of integrated modeling platforms. Agricultural Water Management, 194, 100–112.
Sánchez, A. S., Nogueira, I. B. R., & Kalid, R. A. (2015). Uses of the reject brine from inland desalination for fish farming, Spirulina cultivation, and irrigation of forage shrub and crops. Desalination., 364, 96–107.
Belay, A., Kato, T., & Ota, Y. (1996). Spirulina (Arthrospira): potential application as an animal feed supplement. Journal of Applied Phycology, 8(4-5), 303–311.
Vonshak, A. (1997). Spirulina platensis (Arthrospira): Physiology, Cell Biology and Biotechnology. London: Taylor and Francis.
Silva, C. E. F., & Bertucco, A. (2016). Bioethanol from microalgae and cyanobacteria: A review and technological outlook. Process Biochemistry, 51(11), 1833–1842.
Shirazi, H. M., Sabet, J. K., & Ghotbi, C. (2017). Biodiesel production from Spirulina microalgae feedstock using direct transesterification near supercritical methanol condition. Bioresource Technology, 239, 378–386.
Matos, A. P., Moecke, E. H. S., & Sant’Anna, E. S. (2017). The use of desalination concentrate as a potential substrate for microalgae cultivation in Brazil. Algal Research, 24, 505–508.
Singh, A., & Olsen, S. I. (2011). A critical review of biochemical conversion, sustainability and life cycle assessment of algal biofuels. Applied Energy, 88(10), 3548–3555.
Volkmann, H., Imianovsky, U., Oliveira, J. L. B., & Sant’Anna, E. S. (2007). Cultivation of Arthrospira (Spirulina) platensis in desalinator wastewater and salinated synthetic medium: Protein content and amino-acid profile. Brazilian Journal of Microbiology, 39, 98–101.
Matos, A. P., Feller, R., Moecke, E. H. S., & Sant’Anna, E. S. (2015). Biomass, lipid productivities and fatty acids composition of marine Nannochloropsis gaditana cultured in desalination concentrate. Bioresource Technology, 197, 48–55.
Matos, A. P., Ferreira, W. B., Torres, R. O. C., Morioka, L. R. I., Canella, M. H. M., Rotta, J., Silva, J. T., Moecke, E. H. S., & Sant’Anna, E. S. (2014). Optimization of biomass production of Chlorella vulgaris grown in desalination concentrate. Journal of Applied Phycology, 27, 1473–1483.
Costa, J. A. V., Colla, L. M., & Filho, P. D. (2004). Improving Spirulina platensis biomass yield using a fed-batch process. Bioresource Technology, 92(3), 237–241.
APHA. (2005). Standard Methods for the examination of water and wastewater. Washington DC: American Public Health Association/American Water Works Association/Water Environment Federation.
Morais, M. G., & Costa, J. A. V. (2007). Carbon dioxide fixation by Chlorella kessleri, C. vulgaris, Scenedesmus obliquus and Spirulina sp. cultivated in flasks and vertical tubular photobioreactors. Biotechnology Letters, 29(9), 1349–1352.
Costa, J. A. V., Colla, L. M., Duarte Filho, P., Kabke, P. K., & Weber, A. (2002). Modelling of Spirulina platensis growth in fresh water using response surface methodology, World J. Microbial Biotechnology, 18(7), 603–607.
Lowry, O. H., Rosebrough, N. J., Farr, A. L., & Randall, R. J. (1951). Protein measurement with the Folin phenol reagent. The Journal of Biological Chemistry, 193(1), 265–275.
Dubois, M., Gilles, K. A., Hamilton, J. K., Rebers, P. A., & Smith, F. (1956). Colorimetric method for determination of sugars and related substances. Analytical Chemistry, 28(3), 350–356.
AOAC (Ed.). (1995). Official Methods of Analysis of the association of analytical chemists international (16th ed.). Arlington: AOAC International.
Folch, J., Lees, M., & Stanley, G. H. S. (1957). A simple method for the isolation and purification of total lipids from animal tissues. The Journal of Biological Chemistry, 226(1), 497–509.
Andrade, B. B., Cardoso, L. G., Assis, D. J., Costa, J. A. V., Druzian, J. I., & Lima, S. T. C. (2019). Production and characterization of Spirulina sp. LEB 18 cultured in reused Zarrouk’s medium in a raceway-type bioreactor. Bioresource Technology, 284, 340–348.
Nascimento, I. A., Marques, S. S. I., Cabanelas, I. T. D., Carvalho, G. C., Nascimento, M. A., Souza, C. O., Druzian, J. I., Hussain, J., & Liao, W. (2014). Microalgae versus land crops as feedstock for biodiesel: productivity, quality and standard compliance. Bioenergy Research, 7, 1002–1013.
Freitas, B. C. B., Bracher, E. H., Morais, E. G., Atala, D. I. P., Morais, M. G., & Costa, J. A. V. (2017). Cultivation of different microalgae with pentose as carbon source and the effects on the carbohydrate content. Environmental Technology, 40, 1062–1070.
Rosa, G. M., Moraes, L., Cardias, B. B., Souza, M. R., & Costa, J. A. V. (2015). Chemical absorption and CO2 biofixation via the cultivation of Spirulina in semicontinuous mode with nutrient recycle. Bioresource Technology, 192, 321–327.
Borowitzka, M. A., & Moheimani, N. R. (2013). Sustainable biofuels from algae. Mitigation and Adaptation Strategies for Global Change, 18, 13–25.
Tomaselli, L. (1997). Morphology, ultrastructure and taxonomy of Arthrospira (Spirulina). In A. Vonshak (Ed.), Spirulina platensis (Arthrospira) Physiology, cell biology and biotechnology (pp. 01–16). London: Taylor & Francis.
Leema, J. T. M., Kirubagaran, R., Vinithkumar, N. V., Dheenan, P. S., & Karthikayulu, S. (2010). High value pigment production from Arthrospira (Spirulina) platensis cultured in seawater. Bioresource Technology, 101(23), 9221–9227.
Markou, G., Angelidaki, I., & Georgakakis, D. (2012). Microalgal carbohydrates: an overview of the factors influencing carbohydrates production, and of main bioconversion technologies for production of biofuels. Applied Microbiology and Biotechnology, 96(3), 631–645.
Geider, R. J., & La Roche, J. (2002). Redfield revisited: variability of C:N:P in marine microalgae and its biochemical basis. European Journal of Phycology, 37(1), 1–17.
Raven, J. A., & Beardall, J. (2004). Carbohydrate metabolism and respiration in algae. In A. W. D. Larkum, S. E. Douglas, & J. A. Raven (Eds.), Photosynthesis in algae, Advances in photosynthesis and respiration (pp. 205–224). Dordrecht: Springer.
Li, K., Liu, S., & Liu, X. (2014). An overview of algae bioethanol production. International Journal of Energy Research, 38(8), 965–977.
Ma, Y., Gao, Z., Wang, Q., & Liu, Y. (2018). Biodiesels from microbial oils: Opportunity and challenges. Bioresource Technology, 263, 631–641.
Griffiths, M. J., & Harrison, S. T. (2009). Lipid productivity as a key characteristic for choosing algal species for biodiesel production. Journal of Applied Phycology, 21(5), 493–507.
Paliwal, C., Mitra, M., Bhayani, K., Bharadwaj, A. V. V., Ghosh, T., Dubey, S., & Mishra, S. (2017). Abiotic stresses as tools for metabolites in microalgae. Bioresource Technology, 244(Pt 2), 1216–1226.
Kirst, G. O. (1989). Salinity tolerance of eukaryotic marine algae. Annual Review of Plant Physiology and Plant Molecular Biology, 41, 21–53.
Garcia, J. M. R., Fernández, F. G. A., & Sevilla, J. M. F. (2012). Development of a process for the production of L-amino-acids concentrates from microalgae by enzymatic hydrolysis. Bioresource Technology, 112, 164–170.
Zhang, X., Yuan, H., Jiang, Z., Lin, D., & Zhang, X. (2018). Impact of surface tension of wastewater on biofilm formation of microalgae Chlorella sp. Bioresource Technology, 266, 498–506.
Ye, Y., Huang, Y., Xia, A., Fu, Q., Liao, Q., Zeng, W., Zheng, Y., & Zhu, X. (2018). Optimizing culture conditions for heterotrophic-assisted photoautotrophic biofilm growth of Chlorella vulgaris to simultaneously improve microalgae biomass and lipid productivity. Bioresource Technology, 270, 80–87.
Singh, R., Parihar, P., Singh, M., Badguz, A., Kumar, J., Singh, S., Singh, V. P., & Prasad, S. M. (2017). Uncovering potential applications of cyanobacteria and algal metabolites in biology, agriculture and medicine: current status and future prospects. Frontiers in Microbiology, 8, 1–37.
Fon Sing, S. F., Isdepesky, A., Borowitzka, M. A., & Lewis, D. M. (2014). Pilot scale continuous recycling of growth medium for the mass culture of a halotolerant Tetraselmis sp. in raceway ponds under increasing salinity: a novel protocol for commercial microalgal biomass production. Bioresource Technology, 161, 47–54.
Fujii, S., Uenaka, M., Nakayama, S., Yamamoto, R., & Mantani, S. (2001). Effects of sodium chloride on the fatty acids composition in Boekelovia hooglandii (Ochromonadales, Chrysophyceae). Phycological Research, 49(1), 73–77.
Sun, X. M., Geng, L. J., Ren, L. J., Ji, X. J., Hao, N., Chen, K. Q., & Huang, H. (2018). Influence of oxygen on the biosynthesis of polyunsaturated fatty acids in microalgae. Bioresource Technology, 250, 868–876.
Plouguerné, E., Da, G. B., Pereira, R. C., & Barretobergter, E. (2014). Glycolipids from seaweeds and their potential biotechnological applications. Frontiers in Cellular and Infection Microbiology, 4, 174.
Ruxton, C. H., Calder, P. C., Reed, S. C., & Simpson, M. J. (2005). The impact of long chain n-3 polyunsaturated fatty acids on human health. Nutrition Research Reviews, 18, 113–129.
Kondamudi, N., Strull, J., Misra, M., & Mohapatra, S. (2009). A green process for producing biodiesel from feater meal. Journal of Agricultural and Food Chemistry, 57(14), 6163–6166.
Knothe, G. (2005). Dependence of biodiesel fuel properties on the structure of fatty acid alkyl esters. Fuel Processing Technology, 86, 1059–1070.
Acknowledgments
The authors acknowledge CAPES (Coordination for the Improvement of Higher Education Personnel), MCTIC (Ministry of Science Technology, Innovation and Communications), and the Program to Support the Production of Academic Publications/PROPESP/FURG/2018.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Duarte, J.H., Cardoso, L.G., de Souza, C.O. et al. Brackish Groundwater from Brazilian Backlands in Spirulina Cultures: Potential of Carbohydrate and Polyunsaturated Fatty Acid Production. Appl Biochem Biotechnol 190, 907–917 (2020). https://doi.org/10.1007/s12010-019-03126-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-019-03126-7