Abstract
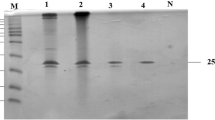
The gene of a β-xylanase (Tnap_0700) was cloned from a hyperthermophilic Thermotoga naphthophila strain ATCC BAA-489 and expressed in Escherichia coli BL21 (DE3) via pET-21a (+) as an expression vector. The growth steps were upgraded for highest β-xylanase expression via several factors, for example, IPTG concentration, time of induction, pH, and temperature. The pH and temperature optima for the extreme expression of β-xylanase were 7.0 pH and 37 °C, correspondingly. Recombinant enzyme purified by heat treatment process, then later by immobilized metal ion affinity chromatography. Molecular mass of the purified β-xylanase was 38 kDa observed by sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE). The enzyme was stable at room temperature for 30 days. It exhibited high stability over wide series of temperature 50–90 °C and pH 4.0–9.0 upon the addition of 1 mM Ca+2 and reduced in the existence of Cu+2 and EDTA. The addition of about 10–30% different organic solvents have no considerable effect on enzyme. However, SDSF and urea acting as an inhibitor leads to decrease in the enzyme activity. The β-xylanase enzyme was active to hydrolyze xylan from beechwood forming xylose. Thermostable β-xylanase causes the breakdown of complex carbohydrates into monosaccharide components. This thermostable β-xylanase revealed remarkable properties, which make it an encouraging candidate for various industrial applications especially in the alteration of renewable biomaterials into ethanol production, and biofuels from lignocellulosics has acknowledged much devotion subsequently in the last decade.




Similar content being viewed by others
References
Fuzi, S. F. Z. M., Mahadi, N. M., Jahim, J. M., Murad, A. M. A., Bakar, F. D. A., Jusoh, M., Rahman, R. A., & Illias, R. M. (2011). Development and validation of a medium for recombinant endo-β-1,4-xylanase production by Kluyveromyces lactis using a statistical experimental design. Annales de Microbiologie, 62(1), 283–292.
Khandeparker, R., Verma, P., & Deobagkar, D. (2011). A novel halotolerant xylanase from marine isolate Bacillus subtilis cho40: gene cloning and sequencing. New Biotechnology, 28(6), 814–821.
Michelin, M., Lourdes, M. D., Polizeli, T. D., Silva, D. P. D., Ruzene, D. S., Vicente, A. A., Jorge, J. A., Terenzi, H. F., & Teixeria, J. A. (2011). Production of xylanolytic enzymes by Aspergillus terricola in stirred tank and airlift tower loop bioreactors. Journal of Industrial Microbiology & Biotechnology, 38(12), 1979–1984.
Rajoka, M. I., Ahmed, S., Hashmi, A. S., & Athar, M. (2012). Production of microbial biomass protein from mixed substrates by sequential culture fermentation of Candida utilis and Brevibacterium lactofermentum. Annales de Microbiologie, 62(3), 1173–1179.
Collins, T., Gerday, C., & Feller, G. (2005). Xylanases, xylanase families and extremophilic xylanases. FEMS Microbiology Reviews, 29(1), 3–23.
Shallom, D., & Shoham, Y. (2003). Microbial hemicellulases. Current Opinion in Microbiology, 6(3), 219–228.
Weng, X. Y., & Sun, J. Y. (2010). Hydrolysis of xylans by a thermostable hybrid xylanase expressed in Escherichia coli. Applied Biochemistry and Microbiology, 46(5), 511–514.
Zhang, J., Siika-aho, M., Puranen, T., Tang, M., Tenkanen, M., & Viikari, L. (2011). Thermostable recombinant xylanases from Nonomuraea flexuosa and Thermoascus aurantiacus show distinct properties in the hydrolysis of xylans and pretreated wheat straw. Biotechnology for Biofuels, 4(1), 12.
Ruller, R., Deliberto, L., Ferreira, T. L., & Ward, R. J. (2008). Thermostable variants of the recombinant xylanase A from Bacillus subtilis produced by directed evolution show reduced heat capacity changes. Proteins, 70(4), 1280–1293 9.
Mardanov, A. V., Svetlitchnyi, V. A., Beletsky, A. V., Prokofeva, M. I., BonchOsmolovskaya, E. A., Ravin, N. V., & Skryabin, K. G. (2010). The genome sequence of the crenarchaeon Acidilobus saccharovorans supports a new order, Acidilobales, and suggests an important ecological role in terrestrial acidic hot Springs. Applied and Environmental Microbiology, 76(16), 5652–5657.
Angelov, A., Liebl, S., Ballschmiter, M., Boemeke, M., Lehmann, R., Liesegang, H., Daniel, R., & Liebl, W. (2010). Genome sequence of the polysaccharide-degrading, thermophilic anaerobe Spirochaeta thermophila DSM 6192. Journal of Bacteriology, 192(24), 6492–6493.
Ahmed, S., Riaz, S., & Jamil, A. (2009). Molecular cloning of fungal xylanases: an overview. Applied Microbiology and Biotechnology, 84(1), 19–35.
Teixeira, R., Siqueira, S., Siqueira, F. G., Souza, M. V. D., Filho, E. X. F., & Bon, E. P. D. S. (2010). Purification and characterization studies of a thermostable β-xylanase from Aspergillus awamori. Journal of Industrial Microbiology & Biotechnology, 37(10), 1041–1051.
Beg, Q. K., Kapoor, M., Mahajan, L., & Hoondal, G. S. (2001). Microbial xylanases and their industrial applications: a review. Applied Microbiology and Biotechnology, 56(3-4), 326–338.
Romdhane, I. B. B., & Achouri, I. M. (2010). Improvement of highly thermostable xylanases production by Talaromyces thermophilus for the agro-industrials residue hydrolysis. Applied Biochemistry and Biotechnology, 162(6), 1635–1646.
Knob, A., & Carmona, E. C. (2010). Purification and characterization of two extracellular xylanases from Penicillium sclerotiorum: a novel acidophilic xylanase. Applied Biochemistry and Biotechnology, 162(2), 429–443.
Sriyapai, T., Somyoonsap, P., Matsui, K., Kawai, F., & Chansin, K. (2011). Cloning of a thermostable xylanase from Actinomadura sp. S14 and its expression in Escherichia coli and Pichia pastoris. Journal of Bioscience and Bioengineering, 111(5), 528–536.
Haltrich, D., Nidetzky, B., Kulbe, K. D., Steiner, W., & Zupancic, S. (1996). Production of fungal xylanases. Bioresource Technology, 58(2), 137–161.
Auria, S. D., Nucci, R., Rossi, M., Gryczynski, I., Gryczynski, Z., & Lakowicz, J. R. (1999). The β-glycosidase from the hyperthermophilic archaeon Sulfolobus solfataricus: enzyme activity and conformational dynamics at temperatures above 100 °C. Biophysical Chemistry, 81(1), 23–31.
Takahata, Y., Nishijima, M., Hoaki, T., & Maruyama, T. (2001). Thermotoga petrophila sp. nov. and Thermotoga naphthophila sp. nov., two hyperthermophilic bacteria from the Kubiki oil reservoir in Niigata, Japan. International Journal of Systematic and Evolutionary Microbiology, 51(5), 1901–1909.
Miller, G. (1959). Use of dinitrosalicylic acid reagent for determination reducing sugar. Analytical Chemistry, 31(3), 426–428.
Bradford, M. M. (1976). A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Analytical Biochemistry, 72(1-2), 248–254.
Laemmeli, U. (1970). Cleavage of structural B proteins during the assembly of the head to bacteriophage T4. Nature, 227(5259), 680–685.
Stephens, D. E., Rumbold, K., Permaul, K., Prior, B. A., & Singh, S. (2007). Directed evolution of the thermostable xylanase from Thermomyces lanuginosus. Journal of Biotechnology, 127(3), 348–354.
Dvorak, P., Chrast, L., Nikel, P. I., Fedr, R., Soucek, K., Sedlackova, M., Chaloupkova, R., Lorenzo, V. D., Prokop, Z., & Damborsky, J. (2015). Exacerbation of substrate toxicity by IPTG in Escherichia coli BL21 (DE3) carrying a synthetic metabolic pathway. Microbial Cell Factories, 14(1), 201.
Amaya-Delgado, L., Mejía-Castillo, T., Santiago-Hernández, A., Vega-Estrada, J., Amelia, F.-G. S., Xoconostle-Cázares, B., Ruiz-Medrano, R., Montes-Horcasitas, M. C., & Hidalgo-Lara, M. E. (2010). Cloning and expression of a novel, moderately thermostable xylanase-encoding gene (Cfl xyn11A) from Cellulomonas flavigena. Bioresource Technology, 101(14), 5539–5545.
Liebl, W., Winterhalter, C., Baumeister, W., Armbrecht, M., & Valdez, M. (2007). Xylanase Attachment to the Cell Wall of the Hyperthermophilic Bacterium Thermotoga maritima. American Society for Microbiology. Journal of Bacteriology https://doi.org/10.1128/JB.01149-07
Dhanjoon, J., Ying, X., Salma, F., & Ma, K. (2013). Characterization of a thermostable xylanase from the extremely thermophilic bacterium Thermotoga hypogea. Current Biotechnology, 2(4), 325–333.
Chunhan, K., Hung, T. C., Jenn, T., YI, L. H., Ting, K. L., An, K. P., & kay, L. Y. (2010). Molecular cloning and characterization of a novel thermostable xylanase from Paenibacillus campinasensis BL11. Process Biotechnology, 45(10), 1638–1644.
Pooma, C. A. (2011). Purification and biochemical characterization of xylanases from Bacillus Pumilus and their potential for hydrolysis of polysaccharides. Journal of Fermentation Technology, 1(1). https://doi.org/10.4172/2167-7972.1000101.
Shi, H., Zhang, Y., LI, X., Huang, Y., Wang, L., Wang, Y., Ding, H., & Wang, F. (2013). Anovel highly thermostable xylanase stimulated by Ca2+ from Thermotoga thermarum: cloning, expression and characterization. Biotechnology for Biofuels, 6(1), 26.
Saanghi, A., Garg, N., Gupta, V. K., Mittal, A., & Kuhad, R. C. (2009). One-step purification and characterization of cellulase-free xylanase produced by alkalophilic Bacillus subtilis ash. Brazilian Journal of Microbiology, 41, 467–476.
Long, L., Xu, M., Shi, Y., Lim, Q., Wang, J., & Ding, S. (2018). Characterization of two new endo-β-1,4-xylanases from Eupenicillium parvum 4-14 and theri applicatins for production of feruloylated oligosaccharides. Applied Biochemistry and Biotechnology, 186(4), 816–833.
Daniel, R. M. (1996). The upper limits of enzyme thermal stability. Enzyme and Microbial Technology, 19(1), 74–79.
Zverlov, V., Piotukh, K., Dakhova, O., Velikodvorskaya, G., & Borriss, R. (1996). The multidomain xylanase A of the hyperthermophilic bacterium Thermotoga neapolitana is extremely thermoresistant. Applied Microbiology and Biotechnology, 45(1-2), 245–247.
Ikram ul, H., Hussain, Z., Khan, M. A., Muneer, B., Afzal, S., Majeed, S., & Akram, F. (2012). Kinetic and thermodynamic study of cloned thermostable endo-1,4-βxylanase from Thermotoga petrophila in mesophilic host. Molecular Biology Reports, 39(7), 7251–7261.
Bouacem, A., Amel, B. D., Nawel, B., Manon, J., Mohammed, G., Wajdi, B. H., Mouloud, K., Said, B., Hocine, H., Bernard, O., & Marie, L. F. (2014). Partial characterization of xylanase produced by Caldicoprobacter algeriensis, a new thermophilic anaerobic bacterium Isolated from an Algerian hot spring. Applied Biochemistry and Biotechnology, 174(5), 1969–1981.
Li, H., Wu, H., Jiang, F., Wu, Y., Xue, J., Gan, L., Liu, J., & Long, M. (2018). Heterologous expression and characterization of an acidic GH11 family xylanase from Hypocrea orientalis. Applied Biochemistry and Biotechnology, 184(1), 228–238.
de Oliveira Simoes, L. C., da Silva, R. R., de Oliveria Nascimento, C. E., Boscolo, M., omes, E., & da Silva, R. (2019). Purification and physicochemical characterization of a novel thermostable xylanase secreted by the fungus Myceliophthora heterothallica F.2.1.4. Applied Biochemistry and Biotechnology. https://doi.org/10.1007/s12010-019-02973-8.
Graciano, L., Juliana, M. C., Fabiola, G. N. V., Adilson, B., Eduardo, A. L., Marina, K. K., & Eduardo, A. L. (2015). Cloning and expression of the xynA1 gene encoding a xylanase of the GH10 group in Caulobacter crescentus. Applied Biochemistry and Biotechnology, 175(8), 3915–3929.
Latif, F., Asgher, M., Saleem, R., Akrem, A., & Legge, R. L. (2006). Purification and characterization of a xylanase produced by Chaetomium thermophile NIBGE. World Journal of Microbiology and Biotechnology, 22(1), 45–50.
Verma, D., & Satyanarayana, T. (2012). Cloning, expression and applicability of thermo-alkali-stable xylanase of Geobacillus thermoleovorans in generating xylooligosaccharides from agro-residues. Bioresource Technology, 107, 333–338.
Chauvaux, S., Souchon, H., Alzari, P. M., Chariot, P., & Beguin, P. (1995). Structural and functional analysis of the metal-binding sites of Clostridium thermocellum endoglucanase CelD. The Journal of Biological Chemistry, 270(17), 9757–9762.
Choi, S. K., & Ljungdahl, L. G. (1996). Structural role of calcium for the organization of the cellulosome of Clostridium thermocellum. Biochemistry, 35(15), 4906–4910.
Spurway, T. D., Morland, C., Cooper, A., Sumner, I., Hazlewood, G. P., O’Donnell, A. G., Pickersgill, R. W., & Gilbert, H. J. (1997). Calcium protects a mesophilic xylanase from proteinase inactivation and thermal unfolding. The Journal of Biological Chemistry, 272(28), 17523–17530.
Do, T., Dam, T., & Quyen, D. (2009). Purification and biophysical characterization of xylanase from Aspergillus niger DSM 1957. Journal of Agriculture and Rural Development in the Tropics and Subtropics, 6, 16–21.
Zafar, A., Aftab, M. N., Din, Z. U., Aftab, S., Iqbal, I., Shahid, A., Tahir, A., & Haq, I. U. (2015). Cloning, expression and purification of xylanase gene from Bacillus licheniformis for use in saccharification of plant biomass. Applied Biochemistry and Biotechnology, 178(2), 294–311. https://doi.org/10.1007/s12010-015-1872-z.
Blei, H. S., Soro, R. Y., Dabonne, S., & Kouame, L. P. (2010). A novel polysaccharidase with endo-beta-D-xylanase and endo-beta-D-glucanase activities in the gut of the major soldier of the termite Macrotermes subhyalinus. Journal of Animal and Plant Science, 8(1), 912–926.
Silva, L. A. O., Terrasan, C. R. F., & Carmona, E. C. (2015). Purification and characterization of xylanases from Trichoderma inhamatum. Electronic Journal of Biotechnology, 18(4), 307–313.
Kamble, R. D., & Jadhav, A. R. (2011). Isolation, purification, and characterization of xylanase produced by a new species of Bacillus in solid state fermentation. International Journal of Microbiology, 2012, 1–8.
Bibi, Z., Ansari, A., Zohra, R. R., Aman, A., & Qader, S. A. U. (2014). Production of xylan degrading endo-1, 4-β-xylanase from thermophilic Geobacillus stearothermophilus KIBGE-IB29. Journal of Radiation Research and Applied Science, 7(4), 478–485.
Todhanakasem, T., Sowatad, A., Kanokratana, P., Havanapan, P., & Champreda, H. (2019). Expression and extracellular secretion of endo-glucanase and xylanase by Zymomonas msobilis. Applied Biochemistry and Biotechnology, 187(1), 239–252.
Van Gool, M. P., van Muiswinkel, G. C., Hinz, S. W., Schols, H. A., Sinitsyn, A. P., & Gruppen, H. (2012). Two GH10 endo-xylanases from Myceliophthora thermophila C1 with and without cellulose binding module act differently towards soluble and insoluble xylans. Bioresource Technology, 119, 123–132.
Van Gool, M. P., van Muiswinkel, G. C., Hinz, S. W., Schols, H. A., Sinitsyn, A. P., & Gruppen, H. (2013). Two novel GH11 endo-xylanases from Myceliophthora thermophila C1 act differently toward soluble and insoluble xylans. Enzyme. Microbiol. Technol., 53(1), 25–32.
Qing, Q., Guo, Q., Zhou, L., He, Y., Wang, L., & Zhang, Y. (2017). Enhancement of in-situ enzymatic saccharification of corn stover by a stepwise sodium hydroxide and organic acid pretreatment. Applied Biochemistry and Biotechnology, 181(1), 350–364.
Acknowledgments
The manuscript is thoroughly read and corrected for English language by Prof. Dr Stephen J. Perkins, Professor Emeritus, Department of Structural and Molecular Biology, Darwin Building, Gover Street, University College of London, UK.
Funding
This work was supported by the Higher Education Commission Pakistan under grant no. and GC University Lahore Ministry of Science and Technology, Government of Pakistan under grant no. 5535 project “Cost effective pilot scale production of bioethanol.”
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Hamid, A., Aftab, M.N. Cloning, Purification, and Characterization of Recombinant Thermostable β-Xylanase Tnap_0700 from Thermotoga naphthophila. Appl Biochem Biotechnol 189, 1274–1290 (2019). https://doi.org/10.1007/s12010-019-03068-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-019-03068-0