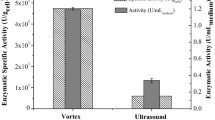
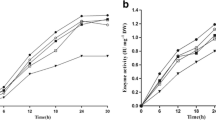
Abstract
In this study, a fungal and two yeast β-galactosidases were immobilized using alginate and chitosan. The biochemical parameters and lactose hydrolysis abilities of immobilized enzymes were analyzed. The pH optima of immobilized fungal β-galactosidases shifted to more acidic pH compared to free enzyme. Remarkably, the optimal temperature of chitosan-entrapped yeast enzyme, Maxilact, increased to 60 °C, which is significantly higher than that of the free Maxilact (40 °C) and other immobilized forms. Chitosan-immobilized A. oryzae β-galactosidase showed improved lactose hydrolysis (95.7%) from milk, compared to the free enzyme (82.7%) in 12 h. Chitosan-immobilized Maxilact was the most efficient in lactose removal from milk (100% lactose hydrolysis in 2 h). The immobilized lactases displayed excellent reusability, and chitosan-immobilized Maxilact hydrolyzed > 95% lactose in milk after five reuses. Compared to free enzymes, the immobilized enzymes are more suitable for cost-effective industrial production of low-lactose milk due to improved thermal activity, lactose hydrolysis efficiencies, and reusability.




Similar content being viewed by others
References
Mlichova, Z., & Rosenberg, M. (2006). Current trends of beta-galactosidase application in food technology. Journal of Food and Nutrition Research, 45(2), 47–54.
Bosso, A., Morioka, L. R. I., dos Santos, L. F., & Suguimoto, H. H. (2016). Lactose hydrolysis potential and thermal stability of commercial β-galactosidase in UHT and skimmed milk. Food Science and Technology (Campinas), 36(1), 159–165. https://doi.org/10.1590/1678-457X.0085.
Demirhan, E., Apar, D. K., & Özbek, B. (2010). A modelling study on hydrolysis of whey lactose and stability of β-galactosidase. Korean Journal of Chemical Engineering, 27(2), 536–545. https://doi.org/10.2478/s11814-010-0062-5.
Katrolia, P., Yan, Q., Jia, H., Li, Y., Jiang, Z., & Song, C. (2011). Molecular cloning and high-level expression of a β-galactosidase gene from Paecilomyces aerugineus in Pichia pastoris. Journal of Molecular Catalysis B: Enzymatic, 69(3–4), 112–119. https://doi.org/10.1016/j.molcatb.2011.01.004.
Martínez-Villaluenga, C., Cardelle-Cobas, A., Corzo, N., Olano, A., & Villamiel, M. (2008). Optimization of conditions for galactooligosaccharide synthesis during lactose hydrolysis by β-galactosidase from Kluyveromyces lactis (Lactozym 3000 L HP G). Food Chemistry, 107(1), 258–264. https://doi.org/10.1016/j.foodchem.2007.08.011.
Katrolia, P., Zhang, M., Yan, Q., Jiang, Z., Song, C., & Li, L. (2011). Characterisation of a thermostable family 42 β-galactosidase (BgalC) family from Thermotoga maritima showing efficient lactose hydrolysis. Food Chemistry, 125(2), 614–621. https://doi.org/10.1016/j.foodchem.2010.08.075.
Niu, D., Tian, X., Mchunu, N. P., Jia, C., Singh, S., Liu, X., Prior, B. A., & Lu, F. (2017). Biochemical characterization of three aspergillus Niger β-galactosidases. Electronic Journal of Biotechnology, 27, 37–43. https://doi.org/10.1016/j.ejbt.2017.03.001.
Oliveira, C., Guimarães, P. M. R., & Domingues, L. (2011). Recombinant microbial systems for improved β-galactosidase production and biotechnological applications. Biotechnology Advances., 29(6), 600–609. https://doi.org/10.1016/j.biotechadv.2011.03.008.
Park, A.-R., & Oh, D.-K. (2010). Galacto-oligosaccharide production using microbial beta-galactosidase: Current state and perspectives. Applied Microbiology and Biotechnology, 85(5), 1279–1286. https://doi.org/10.1007/s00253-009-2356-2.
Dutra Rosolen, M., Gennari, A., Volpato, G., & Volken De Souza, C. F. (2015). Lactose hydrolysis in Milk and dairy whey using microbial β-galactosidases. Enzyme Research, 2015, 1–7. https://doi.org/10.1155/2015/806240.
Kumar Mukesh, D. J., Sudha, M., Devika, S., Balakumaran, M. D., Ravi Kumar, M., & Kalaichelvan, P. T. (2012). Production and optimization of β-galactosidase by Bacillus Sp. MPTK 121, isolated from dairy plant soil. Annals of. Biological Research, 3(4), 1712–1718.
Grosová, Z., Rosenberg, M., & Rebroš, M. (2008). Perspectives and applications of immobilised β-galactosidase in food industry - a review. Czech Journal of Food Sciences., 26(1), 1–14.
Husain, Q. (2010). β galactosidases and their potential applications: A review. Critical Reviews in Biotechnology, 30(1), 41–62. https://doi.org/10.3109/07388550903330497.
Panesar, P. S., Kumari, S., & Panesar, R. (2010). Potential applications of immobilized β-galactosidase in food processing industries. Enzyme Research, 2010(16), 1–16. https://doi.org/10.4061/2010/473137.
DiCosimo, R., McAuliffe, J., Poulose, A. J., & Bohlmann, G. (2013). Industrial use of immobilized enzymes. Chemical Society Reviews, 42(15), 6437. https://doi.org/10.1039/c3cs35506c.
Mohamad, N. R., Marzuki, N. H. C., Buang, N. A., Huyop, F., & Wahab, R. A. (2015). An overview of technologies for immobilization of enzymes and surface analysis techniques for immobized enzymes. Biotechnology and Biotechnological Equipment., 29(2), 205–220. https://doi.org/10.1080/13102818.2015.1008192.
Zhang, D., Yuwen, L., & Peng, L. (2013). Parameters affecting the performance of immobilized enzyme. Journal of Chemistry, 2013(Article ID 946248), 7. https://doi.org/10.1155/2013/946248.
Ansari, S. A., & Satar, R. (2012). Recombinant β-galactosidases - past, present and future: A mini review. Journal of Molecular Catalysis B: Enzymatic., 81, 1–6. https://doi.org/10.1016/j.molcatb.2012.04.012.
Datta, S., Christena, L. R., & Rajaram, Y. R. S. (2013). Enzyme immobilization: An overview on techniques and support materials. 3 Biotech, 3(1), 1–9. https://doi.org/10.1007/s13205-012-0071-7.
Bibi, Z., Qader, S. A. U., & Aman, A. (2015). Calcium alginate matrix increases the stability and recycling capability of immobilized endo-β-1,4-xylanase from Geobacillus stearothermophilus KIBGE-IB29. Extremophiles, 19(4), 819–827. https://doi.org/10.1007/s00792-015-0757-y.
Garcia-Galan, C., Berenguer-Murcia, Á., Fernandez-Lafuente, R., & Rodrigues, R. C. (2011). Potential of different enzyme immobilization strategies to improve enzyme performance. Advanced Synthesis and Catalysis., 353(16), 2885–2904. https://doi.org/10.1002/adsc.201100534.
Ladero, M., Santos, A., & García-Ochoa, F. (2000). Kinetic modeling of lactose hydrolysis with an immobilized β- galactosidase from Kluyveromyces fragilis. Enzyme and Microbial Technology, 27(8), 583–592. https://doi.org/10.1016/S0141-0229(00)00244-1.
Chen, W., Chen, H., Xia, Y., Yang, J., Zhao, J., Tian, F., Zhang, H. P., & Zhang, H. (2009). Immobilization of recombinant thermostable β-galactosidase from Bacillus stearothermophilus for lactose hydrolysis in milk. Journal of Dairy Science, 92(2), 491–498. https://doi.org/10.3168/jds.2008-1618.
Gürdaş, S., Güleç, H. A., & Mutlu, M. (2012). Immobilization of aspergillus oryzae β-galactosidase onto Duolite A568 resin via simple adsorption mechanism. Food and Bioprocess Technology, 5(3), 904–911. https://doi.org/10.1007/s11947-010-0384-7.
Roy, I., & Gupta, M. N. (2003). Lactose hydrolysis by Lactozym ™ immobilized on cellulose beads in batch and fluidized bed modes. Process Biochemistry, 39(3), 325–332. https://doi.org/10.1016/S0032-9592(03)00086-4.
Li, X., Zhou, Q. Z. K., & Chen, X. D. (2007). Pilot-scale lactose hydrolysis using β-galactosidase immobilized on cotton fabric. Chemical Engineering and Processing: Process Intensification, 46(5), 497–500. https://doi.org/10.1016/j.cep.2006.02.011.
Norouzian D.. (2003). Enzyme immobilization: The state of art in biotechnology. Iranian Journal of Biotechnology, 1(4).
Zhang, Z., Zhang, R., Chen, L., & McClements, D. J. (2016). Encapsulation of lactase (β-galactosidase) into k-carrageenan-based hydrogel beads: Impact of environmental conditions on enzyme activity. Food Chemistry, 200(June), 69–75. https://doi.org/10.1016/j.foodchem.2016.01.014.
Funding
This work was supported by National Natural Science Foundation of China (Grant No. 31401628).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Katrolia, P., Liu, X., Li, G. et al. Enhanced Properties and Lactose Hydrolysis Efficiencies of Food-Grade β-Galactosidases Immobilized on Various Supports: a Comparative Approach. Appl Biochem Biotechnol 188, 410–423 (2019). https://doi.org/10.1007/s12010-018-2927-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-018-2927-8