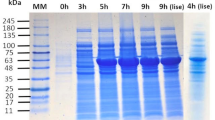
Abstract
The soluble expression of tyrosine decarboxylase (TDC) in heterologous host is often challenging. Here, acidic condition was found to be favorable for improving the soluble expression of TDC from Lactobacillus brevis in Escherichia coli, while addition of carbohydrates (such as glucose, arabinose, and fructose) was vital for decreasing the insoluble fraction. By simple pH control and addition of glucose, the specific activity of TDC in crude extract was enhanced to 46.3 U mg−1, 3.67-fold of that produced from LB medium. Optimization of the reaction conditions revealed that Tween-80 was effective in improving the tyramine production catalyzed by TDC, especially at high tyrosine loadings. As much as 400 mM tyrosine could be completely converted into tyramine with a substrate to catalyst ratio of 29.0 g g−1 and total turnover number of 23,300. This study provides efficient strategies for the highly soluble expression of TDC and biocatalytic production of tyramine.






Similar content being viewed by others
References
Nishimaki-Mogami, T., Suzuki, K., Okochi, E., & Takahashi, A. (1996). Bezafibrate and clofibric acid are novel inhibitors of phosphatidylcholine synthesis via the methylation of phosphatidylethanolamine. Biochimica et Biophysica Acta (BBA)-Lipids and Lipid Metabolism, 1304(1), 11–20. https://doi.org/10.1016/S0005-2760(96)00101-4.
Forman, B. M., Chen, J., & Evans, R. M. (1997). Hypolipidemic drugs, polyunsaturated fatty acids, and eicosanoids are ligands for peroxisome proliferator-activated receptors α and δ. Proceedings of the National Academy of Sciences, 94(9), 4312–4317.
Zhu, Z. T., Munhall, A. C., & Johnson, S. W. (2007). Tyramine excites rat subthalamic neurons in vitro by a dopamine-dependent mechanism. Neuropharmacology, 52(4), 1169–1178. https://doi.org/10.1016/j.neuropharm.2006.12.005.
Lee, K., Kang, K., Park, M., Park, S., & Back, K. (2009). Enhanced octopamine synthesis through the ectopic expression of tyrosine decarboxylase in rice plants. Plant Science, 176(1), 46–50. https://doi.org/10.1016/j.plantsci.2008.09.006.
Roeder, T., Seifert, M., Kahler, C., & Gewecke, M. (2003). Tyramine and octopamine: Antagonistic modulators of behavior and metabolism. Archives of Insect Biochemistry and Physiology, 54(1), 1–13. https://doi.org/10.1002/arch.10102.
Molaei, G., Paluzzi, J. P., Bendena, W. G., & Lange, A. B. (2005). Isolation, cloning, and tissue expression of a putative octopamine/tyramine receptor from locust visceral muscle tissues. Archives of Insect Biochemistry and Physiology, 59(3), 132–149. https://doi.org/10.1002/arch.20067.
Stohs, S. J. (2011). Synephrine: From trace concentrations to massive consumption in weight-loss. Food and Chemical Toxicology, 49(1), 1472–1473. https://doi.org/10.1016/j.fct.2011.03.035.
Mao, G. X., Deng, H. B., Yuan, L. G., Li, D. D., Li, Y. Y., & Wang, Z. (2010). Protective role of salidroside against aging in a mouse model induced by D-galactose. Biomedical and Environmental Sciences, 23(2), 161–166. https://doi.org/10.1016/S0895-3988(10)60047-5.
Ouyang, J. F., Lou, J., Yan, C., Ren, Z. H., & Qiao, H. X. (2010). In-vitro promoted differentiation of mesenchymal stem cells towards hepatocytes induced by salidroside. Journal of Pharmacy and Pharmacology, 62(4), 530–538. https://doi.org/10.1211/jpp.62.04.0017.
Li, Y., Yang, F., Xu, X., Pan, S., Wang, L., & Xia, C. Q. (2009). Improved preparation of tyramine by curtius rearrangement. Chinese Journal of Chemistry, 27(2), 433–436. https://doi.org/10.1002/cjoc.200990072.
Kirk, K. L. (1976). Photochemistry of diazonium salts. 4. Synthesis of ring-fluorinated tyramines and dopamines. Journal of Organic Chemistry, 41(14), 2373–2376. https://doi.org/10.1021/jo00876a004.
Buck, J. S. (1933). Reduction of hydroxy mandelonitrile. A new synthesis of tyramine. Journal of American Chemical Society, 55(8), 3388–3390. https://doi.org/10.1021/ja01335a058.
Wang, Y., & Xie, D. (1994). An improved synthetic method of tyramine. Chinese Journal of Medical Chemistry, 4, 128–129.
Shimizu, Y., Morimoto, H., Zhang, M., & Ohshima, T. (2012). Microwave-assisted deacylation of unactivated amides using ammonium-salt-accelerated transamidation. Angewandte Chemie International Edition, 51(34), 8564–8567. https://doi.org/10.1002/anie.201202354.
Sandmeier, E., Hale, T. I., & Christen, P. (1994). Multiple evolutionary origin of pyridoxal-5′-phosphate-dependent amino acid decarboxylases. European Journal of Biochemistry, 221(3), 997–1002. https://doi.org/10.1111/j.1432-1033.1994.tb18816.x.
Facchini, P. J., & De Luca, V. (1995). Expression in Escherichia coli and partial characterization of two tyrosine/dopa decarboxylases from opium poppy. Phytochemistry, 38(5), 1119–1126. https://doi.org/10.1016/0031-9422(94)00814-A.
El-Ahmady, S. H., & Nessler, C. L. (2001). Cellular localization of tyrosine decarboxylase expression in transgenic opium poppy and tobacco. Plant Cell Reports, 20(4), 313–317. https://doi.org/10.1007/s002990100331.
Ma, L. Q., Gao, D. Y., Wang, Y. N., Wang, H. H., Zhang, J. X., Pang, X. B., Hu, T. S., Lu, S. Y., Li, G. F., Ye, H. C., Li, Y. F., & Wang, H. (2008). Effects of overexpression of endogenous phenylalanine ammonia-lyase (PALrs1) on accumulation of salidroside in Rhodiola sachalinensis. Plant Biology, 10(3), 323–333. https://doi.org/10.1111/j.1438-8677.2007.00024.x.
Zhang, J. X., Ma, L. Q., Yu, H. S., Zhang, H., Wang, H. T., Qin, Y. F., Shi, G. L., & Wang, Y. N. (2011). A tyrosine decarboxylase catalyzes the initial reaction of the salidroside biosynthesis pathway in Rhodiola sachalinensis. Plant Cell Reports, 30(8), 1443–1453. https://doi.org/10.1007/s00299-011-1053-7.
Ishida, Y., & Ozaki, M. (2012). Aversive odorant causing appetite decrease downregulates tyrosine decarboxylase gene expression in the olfactory receptor neuron of the blowfly, Phormia regina. Naturwissenschaften, 99(1), 71–75. https://doi.org/10.1007/s00114-011-0865-1.
Kezmarsky, N. D., Xu, H., Graham, D. E., & White, R. H. (2005). Identification and characterization of a L-tyrosine decarboxylase in Methanocaldococcus jannaschii. Biochimica et Biophysica Acta, 1722(2), 175–182. https://doi.org/10.1016/j.bbagen.2004.12.003.
Borresen, T., Klausen, N. K., Larsen, L. M., & Sørensen, H. (1989). Purification and characterisation of tyrosine decarboxylase and aromatic-L-amino-acid decarboxylase. Biochimica et Biophysica Acta, 993(1), 108–115. https://doi.org/10.1016/0304-4165(89)90149-9.
Lucas, P., & Lonvaud-Funel, A. (2002). Purification and partial gene sequence of the tyrosine decarboxylase of Lactobacillus brevis IOEB 9809. FEMS Microbiology Letters, 211(1), 85–89. https://doi.org/10.1111/j.1574-6968.2002.tb11207.x.
Moreno-Arribas, V., & Lonvaud-Funel, A. (2001). Purification and characterization of tyrosine decarboxylase of Lactobacillus brevis IOEB 9809 isolated from wine. FEMS Microbiology Letters, 195(1), 103–107. https://doi.org/10.1111/j.1574-6968.2001.tb10505.x.
Darbinyan, V., Aslanyan, G., Amroyan, E., Gabrielyan, E., Malmström, C., & Panossian, A. (2007). Clinical trial of Rhodiola rosea L. extract SHR-5 in the treatment of mild to moderate depression. Nordic Journal of Psychiatry, 61(5), 343–348. https://doi.org/10.1080/08039480701643290.
Zhang, K., & Ni, Y. (2014). Tyrosine decarboxylase from Lactobacillus brevis: Soluble expression and characterization. Protein Expression and Purification, 94, 33–39. https://doi.org/10.1016/j.pep.2013.10.018.
Nomura, M., Kobayashi, M., & Ohmomo, S. (2000). Inactivation of the glutamate decarboxylase gene Lactococcus lactis subsp. cremoris. Applied Environmental Microbiology, 66(5), 2235–2237. https://doi.org/10.1128/AEM.66.5.2235-2237.2000.
Baneyx, F., & Mujacic, M. (2004). Recombinant protein folding and misfolding in Escherichia coli. Nature Biotechnology, 22(11), 1399–1408. https://doi.org/10.1038/nbt1029.
de Marco, A., Deuerling, E., Mogk, A., Tomoyasu, T., & Bukau, B. (2007). Chaperone-based procedure to increase yields of soluble recombinant proteins produced in E. coli. BMC Biotechnology, 7(1), 32–40. https://doi.org/10.1186/1472-6750-7-32.
Zhang, H. J., Wei, Y., Lu, Y., Wu, S. P., Liu, Q., Liu, J. Z., & Jiao, Q. C. (2016). Three-step biocatalytic reaction using whole cells for efficient production of tyramine from keratin acid hydrolysis wastewater. Applied Microbiology and Biotechnology, 100(4), 1691–1700. https://doi.org/10.1007/s00253-015-7054-7.
Funding
The study was financially supported by the National Natural Science Foundation of China (21506073, 21776112), the Natural Science Foundation of Jiangsu Province (BK20150003, BK20171135), six talent peaks project of Jiangsu Province (2015-SWYY-008), national first-class discipline program of Light Industry Technology and Engineering (LITE2018-07), the Program of Introducing Talents of Discipline to Universities (111-2-06), and a project funded by the Priority Academic Program Development of Jiangsu Higher Education Institutions.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflicts of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 11450 kb)
Rights and permissions
About this article
Cite this article
Jiang, M., Xu, G., Ni, J. et al. Improving Soluble Expression of Tyrosine Decarboxylase from Lactobacillus brevis for Tyramine Synthesis with High Total Turnover Number. Appl Biochem Biotechnol 188, 436–449 (2019). https://doi.org/10.1007/s12010-018-2925-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-018-2925-x