Abstract
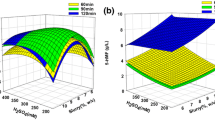
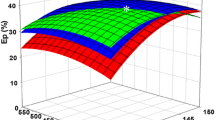
Gracilaria verrucosa, red seaweed, is a promising biomass for bioethanol production due to its high carbohydrate content. The optimal hyper thermal (HT) acid hydrolysis conditions are 12% (w/v) G. verrucosa with 0.2 M H2SO4 at 130 °C for 15 min, with a severity factor of 1.66. This HT acid hydrolysis produces 50.7 g/L monosaccharides. The maximum monosaccharide concentration of 58.0 g/L was achieved with 96.6% of the theoretical monosaccharide production from 120 g dry weight/L G. verrucosa slurry after HT acid hydrolysis and enzymatic saccharification. Fermentation was carried out by removing an inhibitory compound and via yeast adaptation to galactose. Both Pichia stipitis and Kluyveromyces marxianus adapted to galactose were excellent producers, with the ethanol yield (YEtOH) of 0.50 and 29.0 g/L ethanol production. However, the bioethanol productivity with Pichia stipitis adapted to galactose is higher than that with Kluyveromyces marxianus adapted to galactose, being 0.81 and 0.35 g/L/h, respectively. The results from this study can be applied to industrial scale bioethanol production from seaweed.






Similar content being viewed by others
References
Jeong, T. S., Choi, C. H., Lee, J. Y., & Oh, K. K. (2012). Behaviors of glucose decomposition during acid-catalyzed hydrothermal hydrolysis of pretreated Gelidium amansii. Bioresource Technology, 116, 435–440.
McHugh, D. J. (2003). A guide to the seaweed industry. Food and Agriculture Organization of the United Nations.
Yanagisawa, M., Kawai, S., & Murata, K. (2013). Strategies for the production of high concentrations of bioethanol from seaweeds: production of high concentrations of bioethanol from seaweeds. Bioengineered, 4(4), 224–235.
Bensah, E. C., & Mensah, M. (2013). Chemical pretreatment methods for the production of cellulosic ethanol: technologies and innovations. International Journal of Chemical Engineering, 2013, 1–21.
Wyman, C. E., & Yang, B. (2017). Combined severity factor for predicting sugar recovery in acid-catalyzed pretreatment followed by enzymatic hydrolysis. In H. A. Ruiz, M. Thomsen, & H. L. Trajano (Eds.), Hydrothermal processing in biorefineries (pp. 161–180). Cham: Springer International Publishing.
Samuel, R., Pu, Y., Foston, M., & Ragauskas, A. J. (2010). Solid-state NMR characterization of switchgrass cellulose after dilute acid pretreatment. Biofuels, 1(1), 85–90.
Qi, L., Mui, Y. F., Lo, S. W., Lui, M. Y., Akien, G. R., & Horváth, I. T. (2014). Catalytic conversion of fructose, glucose, and sucrose to 5-(hydroxymethyl)furfural and levulinic and formic acids in γ-valerolactone as a green solvent. ACS Catalysis, 4(5), 1470–1477.
Delgenes, J. P., Moletta, R., & Navarro, J. M. (1996). Effects of lignocellulose degradation products on ethanol fermentations of glucose and xylose by Saccharomyces cerevisiae, Zymomonas mobilis, Pichia stipitis, and Candida shehatae. Enzyme and Microbial Technology, 19(3), 220–225.
Lenihan, P., Orozco, A., O’Neill, E., Ahmad, M. N. M., Rooney, D. W., & Walker, G. M. (2010). Dilute acid hydrolysis of lignocellulosic biomass. Chemical Engineering Journal, 156(2), 395–403.
Prakasham, R. S., Rao, R. S., & Hobbs, P. J. (2009). Current trends in biotechnological production of xylitol and future prospects. Current Trends in Biotechnology and Pharmacy, 3(1), 8–36.
Lane, M. M., & Morrissey, J. P. (2010). Kluyveromyces marxianus: a yeast emerging from its sister’s shadow. Fungal Biology Reviews, 24(1-2), 17–26.
Lopez, C. L. F., Beaufort, S., Brandam, C., & Taillandier, P. (2014). Interactions between Kluyveromyces marxianus and Saccharomyces cerevisiae in tequila must type medium fermentation. World Journal of Microbiology and Biotechnology, 30(8), 2223–2229.
Ra, C. H., Kim, Y. J., Lee, S. Y., Jeong, G.-T., & Kim, S.-K. (2015). Effects of galactose adaptation in yeast for ethanol fermentation from red seaweed, Gracilaria verrucosa. Bioprocess and Biosystems Engineering, 38(9), 1715–1722.
Cho, H., Ra, C. H., & Kim, S.-K. (2014). Ethanol production from the seaweed Gelidium amansii, using specific sugar acclimated yeasts. Journal of Microbiology and Biotechnology, 24(2), 264–269.
Ra, C. H., Choi, J. G., Kang, C.-H., Sunwoo, I. Y., Jeong, G.-T., & Kim, S.-K. (2015). Thermal acid hydrolysis pretreatment, enzymatic saccharification and ethanol fermentation from red seaweed, Gracilaria verrucosa. Microbiology and Biotechnology Letters, 43(1), 9–15.
Kubicek, C. P. (1982). Beta-glucosidase excretion by Trichoderma pseudokoningii: correlation with cell wall bound beta-1.3-glucanase activities. Archives of Microbiology, 132(4), 349–354.
Mandels, M., Andreotti, R., & Roche, C. (1976). Measurement of saccharifying cellulase. Biotechnology and Bioengineering Symposium, 6, 21–33.
Fonseca, G. G., Gombert, A. K., Heinzle, E., & Wittmann, C. (2007). Physiology of the yeast Kluyveromyces marxianus during batch and chemostat cultures with glucose as the sole carbon source. FEMS Yeast Research, 7(3), 422–435.
Meinita, M. D. N., Marhaeni, B., Winanto, T., Setyaningsih, D., & Hong, Y.-K. (2015). Catalytic efficiency of sulfuric and hydrochloric acids for the hydrolysis of Gelidium latifolium (Gelidiales, Rhodophyta) in bioethanol production. Journal of Industrial and Engineering Chemistry, 27, 108–114.
Ra, C. H., Nguyen, T. H., Jeong, G.-T., & Kim, S.-K. (2016). Evaluation of hyper thermal acid hydrolysis of Kappaphycus alvarezii for enhanced bioethanol production. Bio/Technology, 209, 66–72.
Meinita, M. D. N., Hong, Y.-K., & Jeong, G.-T. (2012). Detoxification of acidic catalyzed hydrolysate of Kappaphycus alvarezii (cottonii). Bioprocess and Biosystems Engineering, 35(1–2), 93–98.
Kim, D.-H., Lee, S.-B., & Jeong, G.-T. (2014). Production of reducing sugar from Enteromorpha intestinalis by hydrothermal and enzymatic hydrolysis. Bioresource Technology, 161, 348–353.
Kwon, O.-M., Kim, D.-H., Kim, S.-K., & Jeong, G.-T. (2016). Production of sugars from macro-algae Gracilaria verrucosa using combined process of citric acid-catalyzed pretreatment and enzymatic hydrolysis. Algal Research, 13, 293–297.
Nguyen, T. H., Ra, C. H., Sunwoo, I. Y., Jeong, G.-T., & Kim, S.-K. (2016). Evaluation of galactose adapted yeasts for bioethanol fermentation from Kappaphycus alvarezii hydrolyzates. Journal of Microbiology and Biotechnology, 26(7), 1259–1266.
Asztalos, A., Daniels, M., Sethi, A., Shen, T., Langan, P., Redondo, A., & Gnanakaran, S. (2012). A coarse-grained model for synergistic action of multiple enzymes on cellulose. Biotechnology for Biofuels, 5(1), 1–15.
Cannella, D., Hsieh, C. C., Felby, C., & Jørgensen, H. (2012). Production and effect of aldonic acids during enzymatic hydrolysis of lignocellulose at high dry matter content. Biotechnology for Biofuels, 5(1), 1–10.
Guo, Z., & Olsson, L. (2014). Physiological response of Saccharomyces cerevisiae to weak acids present in lignocellulosic hydrolysate. FEMS Yeast Research, 14(8), 1234–1248.
López-Alvarez, A., Díaz-Pérez, A. L., Sosa-Aguirre, C., Macías-Rodríguez, L., & Campos-García, J. (2012). Ethanol yield and volatile compound content in fermentation of agave must by Kluyveromyces marxianus UMPe-1 comparing with Saccharomyces cerevisiae baker’s yeast used in tequila production. Journal of Bioscience and Bioengineering, 113(5), 614–618.
Funding
This research was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF), funded by the Ministry of Education (2016R1D1A1A09918683), Korea.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
About this article
Cite this article
Sukwong, P., Sunwoo, I.Y., Lee, M.J. et al. Application of the Severity Factor and HMF Removal of Red Macroalgae Gracilaria verrucosa to Production of Bioethanol by Pichia stipitis and Kluyveromyces marxianus with Adaptive Evolution. Appl Biochem Biotechnol 187, 1312–1327 (2019). https://doi.org/10.1007/s12010-018-2888-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-018-2888-y