Abstract
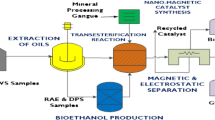
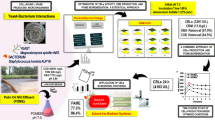
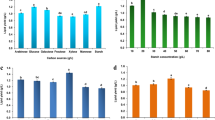
The economical production of lipids is considered as an appropriate renewable alternative feedstock for biodiesel production because of the contemporary concerns on fuel crisis, climate change and food security. In this study, lipid accumulation potential of a novel oleaginous yeast isolate Naganishia liquefaciens NITTS2 by utilizing pre-digested municipal waste activated sludge (PWAS) was explored. Optimization of culture conditions was performed using response surface methodology coupled with genetic algorithm and maximum lipid content of 55.7% was obtained. The presence of lipid was visually confirmed by fluorescence microscopy and its characteristic profile was determined by GC-MS. The yeast lipid was recovered and converted into biodiesel by garbage lipase with the efficiency of 88.34 ± 1.2%, which was further analyzed by proton nuclear magnetic resonance spectroscopy. Hence, the results of this study strongly suggest the possibility of using PWAS as an efficient and low-cost resource for the production of biodiesel from the oleaginous yeast.







Similar content being viewed by others
References
Luque, R., Lovett, J. C., Datta, B., Clancy, J., Campelo, J. M., & Romeroa, A. A. (2010). Biodiesel as feasible petrol fuel replacement: a multidisciplinary overview. Energy & Environmental Science, 3, 1706–1721. https://doi.org/10.1039/C0EE00085J.
Zhang, X., Yan, S., Tyagi, R. D., & Surampalli, R. Y. (2013). Energy balance and greenhouse gas emissions of biodiesel production from oil derived from wastewater and wastewater sludge. Renewable Energy, 55, 392–403. https://doi.org/10.1016/j.renene.2012.12.046.
Lal, B., & Sarma, P. (2009). Wealth from waste trends and technologies. TERI Press.
Venkatesagowda, B., Ponugupaty, E., Barbosa-Dekker, A. M., & Dekker, R. F. H. (2017). The purification and characterization of lipases from Lasiodiplodia theobromae, and their immobilization and use for biodiesel production from coconut oil. Applied Biochemistry and Biotechnology. https://doi.org/10.1007/s12010-017-2670-6.
Poppe, J. K., Matte, C. R., Fernandez-Lafuente, R., Rodrigues, R. C., & Ayub, M. A. Z. (2018). Transesterification of waste frying oil and soybean oil by combi-lipases under ultrasound-assisted reactions. Applied Biochemistry and Biotechnology. https://doi.org/10.1007/s12010-018-2763-x.
Karatay, S. E., & Donmez, G. (2010). Improving the lipid accumulation properties of the yeast cells for biodiesel production using molasses. Bioresource Technology, 101(20), 7988–7990. https://doi.org/10.1016/j.biortech.2010.05.054.
Kwon, E. E., Kim, S., Jeon, Y. J., & Yim, H. (2012). Biodiesel production from sewage sludge: new paradigm for mining energy from municipal hazardous material. Environmental Science and Technology, 46(18), 10222–10228. https://doi.org/10.1021/es3019435.
Sitepu, I. R., Garay, L. A., Sestric, R., Levin, D., Block, D. E., Germanm, J. B., & Boundy-Mills, K. L. (2014). Oleaginous yeasts for biodiesel: current and future trends in biology and production. Biotechnology Advances, 32(7), 1336–1360. https://doi.org/10.1016/j.biotechadv.2014.08.003.
Lopes da Silva, T., Feijao, D., Roseiro, J. C., & Reism, A. (2011). Monitoring Rhodotorula glutinis CCMI 145 physiological response and oil production growing on xylose and glucose using multi-parameter flow cytometry. Bioresource Technology, 102(3), 2998–3006. https://doi.org/10.1016/j.biortech.2010.10.008.
Botham, P. A., & Ratledge, C. A. (1979). A biochemical explanation for lipid accumulation in Candida 107 and other oleaginous micro-organisms. Journal of General Microbiology, 114(2), 361–375. https://doi.org/10.1099/00221287-114-2-361.
He, M. Q., Hu, X., Gou, X., Liu, Q., Li, K., Pan, Q., & Zhu Wu, J. (2010). Screening of oleaginous yeast with xylose assimilating capacity for lipid and bio-ethanol production. African Journal of Biotechnology, 9, 8392–8397.
Probst, K. V., Schulte, L. R., Durrett, T. P., Rezac, M. E., & Vadlani, P. V. (2015). Oleaginous yeast: a value-added platform for renewable oils. Critical Reviews in Biotechnology, 36(5), 942–955. https://doi.org/10.3109/07388551.2015.1064855.
Sanchez, M. A., Ceron Garcia, M. C., Contreras Gomez, A., Garcia Camacho, F., Molina Grima, E., & Chisti, Y. (2003). Shear stress tolerance and biochemical characterization of Phaeodactylum tricornutum in quasi steady-state continuous culture in outdoor photobioreactors. Biochemical Engineering Journal, 16(3), 287–297. https://doi.org/10.1016/S1369-703X(03)00072-X.
Peccia, J., & Westerhoff, P. (2015). We should expect more out of our sewage sludge. Environmental Science and Technology, 49(14), 8271–8276. https://doi.org/10.1021/acs.est.5b01931.
Carrere, H., Dumas, C., Battimelli, A., Batstone, D. J., Delgenes, J. P., Steyer, J. P., & Ferrer, I. (2010). Pretreatment methods to improve sludge anaerobic degradability: a review. Journal of Hazardous Materials, 183(1–3), 1–15. https://doi.org/10.1016/j.jhazmat.2010.06.129.
Selvakumar, P., & Sivashanmugam, P. (2018). Multi-hydrolytic biocatalyst from organic solid waste and its application in municipal waste activated sludge pre-treatment towards energy recovery. Process Safety and Environmental Protection, 117, 1–10. https://doi.org/10.1016/j.psep.2018.03.036.
Leiva-Candia, D. E., Pinzi, S., Redel-Macias, M. D., Koutinas, A., Colin Webb, C., & Dorado, M. P. (2014). The potential for agro-industrial waste utilization using oleaginous yeast for the production of biodiesel. Fuel, 123, 33–42. https://doi.org/10.1016/j.fuel.2014.01.054.
Soufi, M. D., Ghobadian, B., Najafi, G., Mousavi, S. M., & Aubin, J. (2017). Optimization of methyl ester production from waste cooking oil in a batch tri-orifice oscillatory baffled reactor. Fuel Processing Technology, 167, 641–647. https://doi.org/10.1016/j.fuproc.2017.07.030.
Selvakumar, P., Kavitha, S., & Sivashanmugam, P. (2018). Optimization of process parameters for efficient bioconversion of thermo-chemo pretreated Manihot esculenta crantz YTP1 stem to ethanol. Waste and Biomass Valorization. https://doi.org/10.1007/s12649-018-0244-7.
Anand, K., & Elangovan, S. (2017). Optimizing the ultrasonic inserting parameters to achieve maximum pull-out strength using response surface methodology and genetic algorithm integration technique. Measurement, 99, 145–154. https://doi.org/10.1016/j.measurement.2016.12.025.
Yanh, Y., Yan, M., & Hu, B. (2014). Endophytic fungal strains of soybean for lipid production. Bioenergy Research, 7(1), 353–361. https://doi.org/10.1007/s12155-013-9377-5.
Selvakumar, P., & Sivashanmugam, P. (2017). Thermo-chemo-sonic pre-digestion of waste activated sludge for yeast cultivation to extract lipids for biodiesel production. Journal of Environmental Management, 198(1), 90–98. https://doi.org/10.1016/j.jenvman.2017.04.064.
Mouget, J. L., Rosa, P., & Tremblin, G. (2004). Acclimation of Haslea ostrearia to light of different spectral qualities confirmation of chromatic adaptation in diatoms. Journal of Photochemistry and Photobiology B: Biology, 75(1–2), 1–11. https://doi.org/10.1016/j.jphotobiol.2004.04.002.
Zhang, X., Yan, S., Tyagi, R. D., Drogui, P., & Surampalli, R. Y. (2014). Ultrasonication assisted lipid extraction from oleaginous microorganisms. Bioresource Technology, 158, 253–261. https://doi.org/10.1016/j.biortech.2014.01.132.
Selvakumar, P., & Sivashanmugam, P. (2017). Optimization of lipase production from organic solid waste by anaerobic digestion and its application in biodiesel production. Fuel Processing Technology, 165, 1–8. https://doi.org/10.1016/j.fuproc.2017.04.020.
Vicentea, G., Bautista, L. F., Rodriguez, R., Gutierrez, F. J., Sadaba, I., Ruiz-Vazquezb Ros, M., Torres-Martinez, S., & Garre, V. (2009). Biodiesel production from biomass of an oleaginous fungus. Biochemical Engineering Journal, 48(1), 22–27. https://doi.org/10.1016/j.bej.2009.07.014.
DuBois, M., Gilles, K. A., Hamilton, J. K., Rebers, P. A., & Smith, F. (1956). Colorimetric method for determination of sugars and related substances. Analytical Chemistry, 28(3), 350–356.
Meng, X., Yang, J., Xu, X., Zhang, L., Nie, Q., & Xian, M. (2009). Biodiesel production from oleaginous microorganisms. Renewable Energy, 34(1), 1–5. https://doi.org/10.1016/j.renene.2008.04.014.
Beopoulos, A., Cescut, J., Haddouche, R., Uribelarrea, J. L., Molina-Jouve, C., & Nicaud, J. M. (2009). Yarrowia lipolytica as a model for bio-oil production. Progress in Lipid Research, 48(6), 375–387. https://doi.org/10.1016/j.plipres.2009.08.005.
Bruno, W. J., Socci, N. D., & Halpern, A. L. (2000). Weighted neighbor joining: a likelihood-based approach to distance-based phylogeny reconstruction. Molecular Biology and Evolution, 17(1), 189–197. https://doi.org/10.1093/oxfordjournals.molbev.a026231.
Jukes, T. H., & Cantor, C. R. (1969). Evolution of protein molecules. In Mammalian protein metabolism (Vol. 3, pp. 21–132). New York: Academic Press. https://doi.org/10.1016/B978-1-4832-3211-9.50009-7.
Tamura, K., Stecher, G., Peterson, D., Filipski, A., & Kumar, S. (2013). MEGA6: molecular evolutionary genetics analysis version 6.0. Molecular Biology and Evolution, 30(12), 2725–2729.
Griffiths, M. J., & Harrison, S. T. L. (2009). Lipid productivity as a key characteristic for choosing algal species for biodiesel production. Journal of Applied Phycology, 21(5), 493–507. https://doi.org/10.1007/s10811-008-9392-7.
Jawaharraj, K., Karpagam, R., Ashokkumar, B., Kathiresan, S., Moorthy, I. M. G., Arumugam, M., & Varalakshmi, P. (2017). Improved biomass and lipid production in Synechocystis sp. NN using industrial wastes and nano-catalyst coupled transesterification for biodiesel production. Bioresource Technology, 242, 128–132. https://doi.org/10.1016/j.biortech.2017.03.067.
Uma, R. R., Kaliappan, S., Adish Kumar, S., & Rajesh Banu, J. (2012). Combined treatment of alkaline and disperser for improving solubilization and anaerobic biodegradability of dairy waste activated sludge. Bioresource Technology, 126, 107–116. https://doi.org/10.1016/j.biortech.2012.09.027.
Xu, J., Du, W., Zhao, X., Zhang, G., & Liu, D. (2013). Microbial oil production from various carbon sources and its use for biodiesel preparation. Biofuels, Bioproducts and Biorefining, 7(1), 65–77. https://doi.org/10.1002/bbb.1372.
Kock, J. L., & Ratledge, C. (1993). Changes in lipid composition and arachidonic acid turnover during the life cycle of the yeast Dipodascopsis uninucleata. Journal of General Microbiology, 139(3), 459–464. https://doi.org/10.1099/00221287-139-3-459.
Papanikolaou, S., Sarantou, S., Komaitis, M., & Aggelis, G. (2004). Repression of reserve lipid turnover in Cunninghamella echinulata and Mortierella isabellina cultivated in multiple-limited media. Journal of Applied Microbiology, 97(4), 867–875. https://doi.org/10.1111/j.1365-2672.2004.02376.x.
Hansson, L., & Dostalek, M. (1986). Influence of cultivation conditions on lipid production by Cryptococcus albidus. Applied Microbiology and Biotechnology, 24(1), 12–18. https://doi.org/10.1007/BF00266278.
Deeba, F., Pruthi, V., & Negi, Y. S. (2016). Converting paper mill sludge into neutral lipids by oleaginous yeast Cryptococcus vishniaccii for biodiesel production. Bioresource Technology, 213, 96–102. https://doi.org/10.1016/j.biortech.2016.02.105.
Liang, Y., Jarosz, K., Wardlow, A. T., Zhang, J., & Cui, Y. (2014). Lipid production by Cryptococcus curvatus on hydrolysates derived from corn fiber and sweet sorghum bagasse following dilute acid pretreatment. Applied Biochemistry and Biotechnology, 173(8), 2086–2098. https://doi.org/10.1007/s12010-014-1007-y.
Araujo, G. S., Matos, L. J. B. L., Fernandes, J. O., Cartaxo, S. J. M., Gonçalves, L. R. B., Fernandes, F. A. N., & Farias., W. R. L. (2013). Extraction of lipids from microalgae by ultrasound application: prospection of the optimal extraction method. Ultrasonics Sonochemistry, 20(1), 95–98. https://doi.org/10.1016/j.ultsonch.2012.07.027.
Jin, F., Kawasaki, K., Kishida, H., Tohji, K., Moriya, T., & Enomoto, H. (2007). NMR spectroscopy study on methanolysis reaction of vegetable oil. Fuel, 86(7–8), 1201–1207. https://doi.org/10.1016/j.fuel.2006.10.013.
Tariq, M., Ali, S., Ahmad, F., Ahmad, M., Zafar, M., Khalid, N., & Khan, M. A. (2011). Identification, FT-IR, NMR (1H and 13C) and GC/MS studies of fatty acid methyl esters in biodiesel from rocket seed oil. Fuel Processing Technology, 92(3), 336–341. https://doi.org/10.1016/j.fuproc.2010.09.025.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Supplementary data
ESM 1
(DOCX 119 kb)
Rights and permissions
About this article
Cite this article
Selvakumar, P., Sivashanmugam, P. Study on Lipid Accumulation in Novel Oleaginous Yeast Naganishia liquefaciens NITTS2 Utilizing Pre-digested Municipal Waste Activated Sludge: a Low-cost Feedstock for Biodiesel Production. Appl Biochem Biotechnol 186, 731–749 (2018). https://doi.org/10.1007/s12010-018-2777-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-018-2777-4