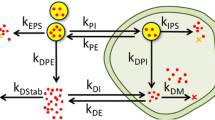
Abstract
Mycobacterium tuberculosis, the causative agent of tuberculosis is now causing death of more than 10 million people. Because of the development of drug-resistant TB, drug delivery to the infected site through nanoparticle had been studied for long time. Nanoparticles indicate different sorts of association with the natural particles of the body. Nanoparticles can be used as controlled or specific drug delivery system. It can be through temporal controlled or can be distribution controlled. Glucose polymer-based nanoparticles might play an important role as drug delivery system in case of targeted drug delivery in the infected site of the body or in infected macrophages, as they are biodegradable so there should not be any side effects of these particles in the body and also they show very slow immune response. CD4, Beta 1, TGFb-1, IL-2, IL-13 SEC14L1, GUSB, BPI, and CCR7 are major biomarkers secreted after infection of this bacterium by the macrophages which can be used for targeted drug delivery in infected macrophages. As these markers can be used for delivery of drugs at destined position, they can be very beneficial in reducing toxicities of antituberculer drugs to the other uninfected sites and in operating only the infected macrophages.
Similar content being viewed by others
Abbreviations
- TB:
-
Tuberculosis
- Mtb :
-
Mycobacterium tuberculosis
- INH:
-
Isoniazid
- RIF:
-
Rifampicin
- PZA:
-
Pyrazinamide
- AMs:
-
Alveolar macrophages
- MIC:
-
Minimal inhibitory concentration
- IFN-γ:
-
Interferon gamma
- IL-2:
-
Interleukin- 2
- CD4:
-
Cluster of differentiation 4
- TGFb-1:
-
Transforming growth factor beta 1
- IL-2:
-
Interleukin
- IL-13:
-
Interleukin
- SEC14L1:
-
Sec14- like lipid binding 1
- GUSB:
-
Glucuronidase beta
- BPI:
-
Bactericidal/permeability-increasing protein
- CCR7:
-
C-C chemokine receptor type 7
References
Zenebe, Y., Anagaw, B., Tesfay, W., Debebe, T., & Gelaw, B. (2013). Smear positive extra pulmonary tuberculosis disease at University of Gondar Hospital, Northwest Ethiopia. BMC., 6, 1–10.
Meena, L. S., & Rajni. (2010). Survival mechanisms of pathogenic Mycobacterium tuberculosis H37Rv. The FEBS Journal, 277(11), 2416–2427. https://doi.org/10.1111/j.1742-4658.2010.07666.x
Nguyen, L., & Pieters, J. (2005). The Trojan horse: survival tactics of pathogenic Mycobacteria in macrophages. Trends in Cell Biology, 15(5), 269–276. https://doi.org/10.1016/j.tcb.2005.03.009
World Health Organization, Global Tuberculosis Report 2014 (WHO, 2014).
World Health Organization, Global Tuberculosis Report 2016 (WHO, 2016).
Chopra, P., Meena, L. S., & Singh, Y. (2003). New drug targets for Mycobacterium tuberculosis. Indian Journal Medical, 117, 1–9.
Collins, H. L., & Kaufmann, S. H. (2001). The many faces of host responses to tuberculosis. Immunol., 103(1), 1–9. https://doi.org/10.1046/j.1365-2567.2001.01236.x
Hamidon, N. H., Suraiya, S., Sarmiento, M. E., Acosta, A., Norazmi, M. N., & Lim, T. S. (2017). Immune TB antibody phage display library as a tool to study B cell immunity in TB infections. Applied Biochemistry and Biotechnology. https://doi.org/10.1007/s12010-017-2582-5
Chen, K., & Kolls, J. K. (2013). T cell-mediated host immune defenses in the lung. Annual Review of Immunology, 31(1), 605–633. https://doi.org/10.1146/annurev-immunol-032712-100019
Schlesinger, L. S. (1993). Macrophage phagocytosis of virulent but not attenuated strains of Mycobacterium tuberculosis is mediated by mannose receptors in addition to complement receptors. Journal of Immunology, 150, 2920–2930.
Kang, P. B., Azad, A. K., Torrelles, J. B., Kaufman, T. M., & Beharka, A. (2005). The human macrophage mannose receptor directs Mycobacterium tuberculosis lipoarabinomannan-mediated phagosome biogenesis. The Journal of Experimental Medicine, 202(7), 987–999. https://doi.org/10.1084/jem.20051239
Cooper, A. M., & Mayer-Barber, K. D. (2011). A role of innate cytokines in mycobacterial infection. Mucosal Immunology, 4(3), 252–260. https://doi.org/10.1038/mi.2011.13
Monu, & Meena, L. S. (2016). Roles of triolein and lipolytic protein in the pathogenesis and survival of Mycobacterium tuberculosis: a novel therapeutic approach. Applied Biochemistry and Biotechnology, 178(7), 1377–1389. https://doi.org/10.1007/s12010-015-1953-z
Chen, L., Guo, S., Wu, L., Fan, X., Ma, H., Wu, K., Wu, J., & Zhang. (2015). IL-17A autoantibody induced by recombinant Mycobacterium smegmatis expressing Ag85A-IL-17A fusion protein. Applied Biochemistry and Biotechnology, 176(7), 2018–2026. https://doi.org/10.1007/s12010-015-1697-9
Philips, J. A. (2008). Mycobacterial manipulation of vacuolar sorting. Cellular Microbiology, 10(12), 2408–2415. https://doi.org/10.1111/j.1462-5822.2008.01239.x
Rodrigues, M. L. (2008). Extracellular vesicles produced by Cryptococcus neoformans contain protein components associated with virulence. Eukaryotic Cell, 7(1), 58–67. https://doi.org/10.1128/EC.00370-07
Clemens, D. L., & Horwitz, M. A. (1995). Characterization of the Mycobacterium tuberculosis phagosome and evidence that phagosomal maturation is inhibited. The Journal of Experimental Medicine, 181(1), 257–270. https://doi.org/10.1084/jem.181.1.257
Kumari, P., & Meena, L. S. (2014). Factors affecting susceptibility to Mycobacterium tuberculosis: a close view of immunological defence mechanism. Applied Biochemistry and Biotechnology, 174(8), 2663–2673. https://doi.org/10.1007/s12010-014-1217-3
Small, P. L., Ramakrishnan, L., & Falkow, S. (1994). Remodeling schemes of intracellular pathogens. Sci., 263(5147), 637–639. https://doi.org/10.1126/science.8303269
He, Q., Zhang, Z., Gao, F., Li, Y., & Shi, J. (2011). In vivo biodistribution and urinary excretion of mesoporous silica nanoparticles: effects of particle size and PEGylation. Small, 7(2), 271–280. https://doi.org/10.1002/smll.201001459
Vanhest, R., Baars, H., Kik, S., van Gerven, P., Trompenaars, M. C., Kalisvaart, N., Keizer, S., Borgdorff, M., Mensen, M., & Cobelens, F. (2004). Hepatotoxicity of rifampin-pyrazinamide and isoniazid preventive therapy and tuberculosis treatment. Clinical Infectious Diseases, 39(4), 488–496. https://doi.org/10.1086/422645
Chen, H., Wang, L., Yeh, J., Wu, X., Cao, Z., Wang, Y. A., Zhang, M., Yang, L., & Mao, H. (2010). Reducing non-specific binding and uptake of nanoparticles and improving cell targeting with an antifouling PEO-b-PγMPS (PEO-b-PγMPS- poly (ethylene oxide)-block-poly (γ-methacryloxypropyltrimethoxysilane)) copolymer coating. Biomaterials, 31, 5397–5407.
He, Q., Gao, Y., Zhang, L., Zhang, Z., Gao, F., Ji, X., Li, Y., & Shi, J. (2011). A pH-responsive mesoporous silica nanoparticles-based multi-drug delivery system for overcoming multi-drug resistance. Biomaterials, 32(30), 7711–7720. https://doi.org/10.1016/j.biomaterials.2011.06.066
Sharma, S., & Meena, L. S. (2017). Potential of Ca2+ in Mycobacterium tuberculosis H37Rv pathogenesis and survival. Applied Biochemistry and Biotechnology, 181(2), 762–771. https://doi.org/10.1007/s12010-016-2247-9
Lee, J. E., Lee, N., Kim, H., Kim, J., Choi, S. H., Kim, J. H., Kim, T., Song, I. C., Park, S. P., Moon, W. K., & Hyeon, T. (2010). Uniform mesoporous dye-doped silica nanoparticles decorated with multiple magnetite nanocrystals for simultaneous enhanced magnetic resonance imaging, fluorescence imaging, and drug delivery. Journal of the American Chemical Society, 132(2), 552–557. https://doi.org/10.1021/ja905793q
Muttil, P., Kaur, J., Kumar, K., Yadav, A. B., Sharma, R., & Misra, A. (2007). Inhalable microparticles containing large payload of anti-tuberculosis drugs. European Journal of Pharmaceutical Sciences, 32(2), 140–150. https://doi.org/10.1016/j.ejps.2007.06.006
Saraogi, G. K., Gupta, P., Gupta, U. D., Jain, N. K., & Agrawal, G. P. (2010). Gelatin nanocarriers as potential vectors for effective management of tuberculosis. International Journal of Pharmaceutics, 385(1-2), 143–149. https://doi.org/10.1016/j.ijpharm.2009.10.004
Olakanmi, O., Stokes, J. B., & Britigan, B. E. (2005). Gallium-inducible transferrin-independent iron acquisition is a property of many cell types: possible role of alterations in the plasma membrane. Journal of Investigative Medicine, 53(3), 143–153. https://doi.org/10.2310/6650.2005.00310
Britigan, B. E., Rasmussen, G. T., Olakanmi, O., & Cox, C. D. (2000). Iron acquisition from Pseudomonas aeruginosa siderophores by human phagocytes: an additional mechanism of host defense through iron sequestration? Inf. and immun., 68(3), 1271–1275. https://doi.org/10.1128/IAI.68.3.1271-1275.2000
Gaddy, J. A., Arivett, B. A., McConnell, M. J., Lopez-Rojas, R., Pachon, J., & Actis, L. A. (2012). Role of acinetobactin-mediated iron acquisition functions in the interaction of Acinetobacter baumannii strain ATCC 19606T with human lung epithelial cells, Galleria mellonella caterpillars, and mice. Infection and immunity, 80(3), 1015–1024. https://doi.org/10.1128/IAI.06279-11
Narayanasamy, P., Switzer, B. L., & Britigan, B. E. (2015). Prolonged-acting, multi-targeting gallium nanoparticles potently inhibit growth of both HIV and mycobacteria in co-infected human macrophages. Scientific Reports, 5(1), 8824. https://doi.org/10.1038/srep08824
Choi, S. R., Britigan, B. E., & Narayanasamy, P. (2017). Ga(III) nanoparticles inhibit growth of both Mycobacterium tuberculosis and HIV and release of interleukin-6 (IL-6) and IL-8 in co-infected macrophages. Antimicrobial agents and chemotherapy., 61(4), e02505–e02516. https://doi.org/10.1128/AAC.02505-16
Choi, S.-r., Britigan, B. E., Moran, D. M., & Narayanasamy, P. (2017). Gallium nanoparticles facilitate phagosome maturation and inhibit growth of virulent Mycobacterium tuberculosis in macrophages. PLoS One, 12(5), e0177987. https://doi.org/10.1371/journal.pone.0177987
Nowacek, A. S., Miller, R. L., McMillan, J., Kanmogne, G., Kanmogne, M., Mosley, R. L., et al. (2009). NanoART synthesis, characterization, uptake, release and toxicology for human monocyte-macrophage drug delivery. Nanomedicine (London, England), 4(8), 903–917. https://doi.org/10.2217/nnm.09.71
Meena, J., Singh, M., Sahare, P. D., & Meena, L. S. (2014). Interaction of nanoparticles in biological systems and their role in therapeutical treatment of tuberculosis and cancer. Journal Luminescene Applications, 1, 7–22.
Saraiva, C., Praça, C., Ferreira, R., Santos, T., Ferreira, L., & Bernardino, L. (2016). Nanoparticle-mediated brain drug delivery: overcoming blood–brain barrier to treat neurodegenerative diseases. Journal of Controlled Release, 235, 34–47. https://doi.org/10.1016/j.jconrel.2016.05.044
Cheng, R., Meng, F., Deng, C., Klok, H. A., & Zhong, Z. (2013). Dual and multi-stimuli responsive polymeric nanoparticles for programmed site-specific drug delivery. Biomaterials, 34, 3647e3657.
Riahi, F., Derakhshan, M., Mosavat, A., Soleimanpour, S., & Rezaee, S. A. (2015). Evaluation of point mutation detection in Mycobacterium tuberculosis with isoniazid resistance using real-time PCR and TaqMan probe assay. Applied Biochemistry and Biotechnology, 175(5), 2447–2455. https://doi.org/10.1007/s12010-014-1442-9
Dang, G., Chen, L., Li, Z., Deng, X., Cui, Y., Cao, J., Yu, S., Pang, H., & Liu, S. (2015). Expression, purification and characterisation of secreted esterase Rv2525c from Mycobacterium tuberculosis. Applied Biochemistry and Biotechnology, 176(1), 1–12. https://doi.org/10.1007/s12010-015-1555-9
Lillebaek, T., Dirksen, A., Vynnycky, E., Baess, I., Thomsen, V. O., & Andersen, A. B. (2003). Stability of DNA patterns and evidence of Mycobacterium tuberculosis reactivation occurring decades after the initial infection. The Journal of Infectious Diseases, 188(7), 1032–1039. https://doi.org/10.1086/378240
Walter, K. A., Tamargo, R., Olivi, A., Burger, P. C., & Brem, H. (1995). Intratumoral chemotherapy. Neurosurgery, 37(6), 1129–1145. https://doi.org/10.1227/00006123-199512000-00013
Wallis, R. S., Pai, M., Menzies, D., Doherty, T. M., Walzl, G., Perkins, M. D., & Zumla, A. (2010). Biomarkers and diagnostics for tuberculosis: progress, needs, and translation into practice. The Lancet, 375(9729), 1920–1937. https://doi.org/10.1016/S0140-6736(10)60359-5
Dhanasekaran, S., Jenum, S., Stavrum, R., Ritz, C., & Jepsen, D. F. (2013). Identification of biomarkers for Mycobacterium tuberculosis infection and disease in BCG-vaccinated young children in Southern India. Genes and Immunity, 14(6), 356–364. https://doi.org/10.1038/gene.2013.26
Funding
The authors acknowledge financial support from the Department of Science and Technology-SERB, Council of Scientific and Industrial Research-Institute of Genomics and Integrative Biology, under the research project GAP0145.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
There authors declare that there is no conflict of interest.
Rights and permissions
About this article
Cite this article
Shivangi, Meena, L.S. A Novel Approach in Treatment of Tuberculosis by Targeting Drugs to Infected Macrophages Using Biodegradable Nanoparticles. Appl Biochem Biotechnol 185, 815–821 (2018). https://doi.org/10.1007/s12010-018-2695-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-018-2695-5