Abstract
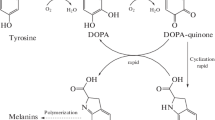
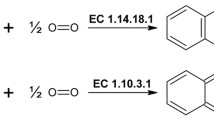
Tyrosinases catalyze oxidation of phenols with a formation of biphenols, quinones, and highly polymerized melanins. Tyrosinases have prospects for industrial use to remove phenols, also in biosensors, in bioorganic synthesis, and for a production of biocompatible adhesives (medical glues). Despite growing fields of potential applications, a selection of commercially available tyrosinases are currently limited to a single enzyme which is isolated from fruiting bodies of mushrooms. This article describes a preparation of recombinant tyrosinase from a bacterium Verrucomicrobium spinosum using a heterologous expression in Escherichia coli. Recombinant V. spinosum tyrosinase has high specific activity (13,200 U/mg). A resistance of the enzyme was investigated to chemical agents used to denature proteins and keep poorly solvable proteins in a solution. The enzyme preserves activity in the presence of urea and retains at least a fraction of its enzymatic activity at concentrations of urea up to 4.5 M. An addition of sodium lauroyl sarcosinate to 1 or 2% activates the tyrosinase. Novel means of quantitatively expressing tyrosinase activity is described in this article. The method uses a set of parameters obtained from non-linear estimation of the progress curves and is suitable for enzymatic reactions which do not comply with Michaelis–Menten kinetics. Tyrosinase may be used to introduce into proteins a post-translational modification which is a conversion of tyrosine residues (Tyr) into residues of 3,4-dioxyphenylalanine (DOPA). The presence of DOPA provides the polypeptides with a capability of strong molecular adhesion. Co-expression of tyrosinase and a recombinant protein mimicking marine mussel-encoded adhesive proteins resulted in obtaining of the protein in which at least a part of Tyr residues had been converted to DOPA. The DOPA-containing protein had high adhesion strength (2.5 MPa).











Similar content being viewed by others
References
Haghbeen, K., & Tan, E. W. (2003). Direct spectrophotometric assay of monooxygenase and oxidase activities of mushroom tyrosinase in the presence of synthetic and natural substrates. Analytical Biochemistry, 312(1), 23–32. https://doi.org/10.1016/S0003-2697(02)00408-6.
Fairhead, M., & Thöny-Meyer, L. (2010). Role of the C-terminal extension in a bacterial tyrosinase. The FEBS Journal, 277(9), 2083–2095. https://doi.org/10.1111/j.1742-4658.2010.07621.x.
Ensuncho, L., Alvarez-Cuenca, M., & Legge, R. L. (2005). Bioprocess Biosyst. Eng, 27, 185–191.
Ikehata, K., & Nicel, J. A. (2000). Bioresour. Technology, 74, 191–199.
Tembe, S., Karve, M., & Inamdar, S. (2006). Development of electrochemical biosensor based on tyrosinase immobilized in composite biopolymeric film. Analytical Biochemistry, 349(1), 72–77. https://doi.org/10.1016/j.ab.2005.11.016.
Nistor, C., Emneus, J., & Gorton, L. (1999). Improved stability and altered selectivity of tyrosinase based graphite electrodes for detection of phenolic compounds. Anal. Chim. Act, 387(3), 309–326. https://doi.org/10.1016/S0003-2670(99)00071-9.
Irimia-Vladu, M. (2014). “Green” electronics: biodegradable and biocompatible materials and devices for sustainable future. Chemical Society Reviews, 43(2), 588–610. https://doi.org/10.1039/C3CS60235D.
Koyanagi, T., Katayama, T., & Suzuki, H. (2005). Effective production of 3,4-dihydroxyphenyl-l-alanine (l-DOPA) with Erwinia herbicola cells carrying a mutant transcriptional regulator TyrR. Journal of Biotechnology, 115(3), 303–306. https://doi.org/10.1016/j.jbiotec.2004.08.016.
Lee, B. P., Messersmith, P., & Israelachvili, J. (2011). Mussel-inspired adhesives and coatings. Annual Review of Materials Research, 41(1), 99–132. https://doi.org/10.1146/annurev-matsci-062910-100429.
Lin, Q., Gourdon, D., & Sun, C. (2007). Adhesion mechanisms of the mussel foot proteins mfp-1 and mfp-3. Proceedings of the National Academy of Sciences of the United States of America, 104(10), 3782–3786. https://doi.org/10.1073/pnas.0607852104.
Lu, Q., Danner, E., Waite, J. H., & Israelachvili, J. N. (2013). J. R. Soc. Interface, 10, 79.
Yu, J., Kan, Y., & Rapp, M. (2013). Adaptive hydrophobic and hydrophilic interactions of mussel foot proteins with organic thin films. Proceedings of the National Academy of Sciences of the United States of America, 110(39), 15680–15685. https://doi.org/10.1073/pnas.1315015110.
Clancy, S. K., Sodano, A., & Cunningham, D. J. (2016). Marine bioinspired underwater contact adhesion. Biomacromolecules, 17(5), 1869–1874. https://doi.org/10.1021/acs.biomac.6b00300.
Li, A., Mu, Y., & Jiang, W. (2015). A mussel-inspired adhesive with stronger bonding strength under underwater conditions than under dry conditions. ChemCommun (Camb), 51(44), 9117–9120. https://doi.org/10.1039/C5CC00101C.
Jeon, E. Y., Hwang, B. H., & Yang, Y. J. (2015). Rapidly light-activated surgical protein glue inspired by mussel adhesion and insect structural crosslinking. Biomaterials, 67, 11–19. https://doi.org/10.1016/j.biomaterials.2015.07.014.
Selinheimo, E., NiEidhin, D., & Steffensen, C. (2007). Comparison of the characteristics of fungal and plant tyrosinases. Journal of Biotechnology, 130(4), 471–480. https://doi.org/10.1016/j.jbiotec.2007.05.018.
Kamal, U., Ayesha, S. Ali & Sharique, A. Ali. (2014). Enzyme Research, 6 pages.
Santos, M. C., Pereira, P. R., Gutarra, M. E., Torres, A. G., Pereira, K. S. & Salgado A. M. (2015). XX Congresso Brasileiro de Engenharia Quimica, 8 pages.
Marková, E., Kotik, M., Křenková, A., Man, P., Haudecoeur, R., Boumendjel, A., Hardré, R., Mekmouche, Y., Courvoisier-Dezord, E., Réglier, M., & Martínková, L. (2016). J. Agric. Food Chemistry, 64(14), 2925–2931. https://doi.org/10.1021/acs.jafc.6b00286.
Zou, Y., Hu, W., Jiang, A., & Ma, K. (2014). Partial purification and characterization of a novel extracellular tyrosinase from Auricularia auricula. Applied Biochemistry and Biotechnology, 172(3), 1460–1469. https://doi.org/10.1007/s12010-013-0638-8.
Inamdar, S., Joshi, S., Bapat, V., & Jadhav, J. (2014). Purification and characterization of RNA allied extracellular tyrosinase from Aspergillus species. Applied Biochemistry and Biotechnology, 172(3), 1183–1193. https://doi.org/10.1007/s12010-013-0555-x.
Aoife, M., Evelyn, M., Doyle, B. S., Kevin, E. & O’Connor. (2007). Enzyme and microbial technology, (40), 1435–1441.
Agarwal, P., Singh, J., & Singh, R. P. (2017). Molecular cloning and characteristic features of a novel extracellular tyrosinase from Aspergillus niger PA2. Applied Biochemistry and Biotechnology, 182(1), 1–15. https://doi.org/10.1007/s12010-016-2306-2.
Lim, S., Choi, Y. S., & Kang, D. G. (2010). The adhesive properties of coacervated recombinant hybrid mussel adhesive proteins. Biomaterials, 31(13), 3715–3722. https://doi.org/10.1016/j.biomaterials.2010.01.063.
Choi, Y. S., Yang, Y. J., & Yang, B. (2012). In vivo modification of tyrosine residues in recombinant mussel adhesive protein by tyrosinase co-expression in Escherichia coli. Microbial Cell Factories, 11(1), 139. https://doi.org/10.1186/1475-2859-11-139.
Yang, B., Kang, D. G., & Seo, J. H. (2013). A comparative study on the bulk adhesive strength of the recombinant mussel adhesive protein fp-3. Biofouling, 29(5), 483–490. https://doi.org/10.1080/08927014.2013.782541.
Yu, J., Wei, W., & Menyo, M. S. (2013). Adhesion of mussel foot protein-3 to TiO2 surfaces: the effect of pH. Biomacromolecules, 14(4), 1072–1077. https://doi.org/10.1021/bm301908y.
Haemers, S., Koper, G. J., & Frens, G. (2003). Effect of oxidation rate on cross-linking of mussel adhesive proteins. Biomacromolecules, 4(3), 632–640. https://doi.org/10.1021/bm025707n.
Monahan, J., & Wilker, J. J. (2004). Cross-linking the protein precursor of marine mussel adhesives: bulk measurements and reagents for curing. Langmuir, 20(9), 3724–3729. https://doi.org/10.1021/la0362728.
Jing, Yu, Yajing, Kan, Michael, Rapp, Danner, E., Wei, W., Saurabh, D., Dusty, R. M., Yunfei, C., Herbert, W. & Jacob, N. (2013). Proc Natl. Acad. Sci. USA, 110(39), 15680–15685.
Funding
This study was funded by a grant from the Ministry of Education and Science of the Republic of Kazakhstan, grant number 0115РК01798.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Axambayeva, A.S., Zhaparova, L.R., Shagyrova, Z.S. et al. Unusual Stability of a Recombinant Verrucomicrobium spinosum Tyrosinase to Denaturing Agents and Its Use for a Production of a Protein with Adhesive Properties. Appl Biochem Biotechnol 185, 736–754 (2018). https://doi.org/10.1007/s12010-017-2686-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-017-2686-y