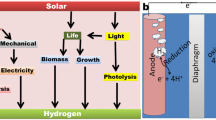
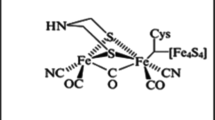
Abstract
Molecular hydrogen is a promising currency in the future energy economy due to the uncertain availability of finite fossil fuel resources and environmental effects from their combustion. It also has important uses in the production of fertilizers and platform chemicals as well as in upgrading conventional fuels. Conventional methods for producing molecular hydrogen from natural gas produce carbon dioxide and use a finite resource as feedstock. However, these issues can be overcome by using light energy from the Sun combined with microorganisms and their molecular machinery capable of photosynthesis. In the presence of light, the proteins involved in photosynthesis coupled with appropriate catalysts in higher plants, algae, and cyanobacteria can produce molecular hydrogen, and optimization via genetic modifications and biomolecular engineering further improves production rates. In this review, we will discuss techniques that have been utilized to improve rates of hydrogen production in biological systems based on the protein machinery of photosynthesis coupled with appropriate catalysts. We will also suggest areas for improvement and future directions for work in the field.




Similar content being viewed by others
References
Hydrogen production: natural gas reforming Available from: https://energy.gov/eere/fuelcells/hydrogen-production-natural-gas-reforming. Accessed 19 March 2017.
Li, C. L., & Fang, H. H. P. (2007). Fermentative hydrogen production from wastewater and solid wastes by mixed cultures. Critical Reviews in Environmental Science and Technology, 37, 1–39.
Iwuchukwu, I. J., Vaughn, M., Myers, N., O’Neill, H., Frymier, P., & Bruce, B. D. (2010). Self-organized photosynthetic nanoparticle for cell-free hydrogen production. Nature Nanotechnology, 5, 73–79.
Pittam, D. A., & Pilcher, G. (1972). Measurements of heats of combustion by flame calorimetry. J. Chem. Soc. Faraday T., 1(68), 2224–2229.
Barber, J. (2012). Photosystem II: the water-splitting enzyme of photosynthesis. Cold Spring Harbor Symposia on Quantitative Biology, 77, 295–307.
Hydrogen storage Available from: https://energy.gov/eere/fuelcells/hydrogen-storage. Accessed 3 May 2017.
Badura, A., Esper, B., Ataka, K., Grunwald, C., Woll, C., Kuhlmann, J., Heberle, J., & Rogner, M. (2006). Light-driven water splitting for (bio-)hydrogen production: photosystern 2 as the central part of a bioelectrochemical device. Photochemistry and Photobiology, 82, 1385–1390.
Sandmann, G., & Boger, P. (1980). Copper-induced exchange of plastocyanin and cytochrome c-533 in cultures of Anabaena variabilis and Plectonema boryanum. Plant Science Letters, 17, 417–424.
Diaz-Quintana, A., Navarro, J. A., Hervas, M., Molina-Heredia, F. P., De la Cerda, B., & De la Rosa, M. A. (2003). A comparative structural and functional analysis of cyanobacterial plastocyanin and cytochrome c6 as alternative electron donors to photosystem I. Photosynthesis Research, 75, 97–110.
Hervas, M., Navarro, J. A., & De la Rosa, M. A. (2003). Electron transport between membrance complexes and soluble proteins in photosynthesis. Accounts of Chemical Research, 36, 798–805.
Li, M., Semchonok, D. A., Boekema, E. J., & Bruce, B. D. (2014). Characterization and evolution of tetrametic photosystem I from the thermophilic cyanobacterium Chroococcidiopsis sp TS-821. Plant Cell, 26, 1230–1245.
Mazor, Y., Borovikova, A., & Nelson, N. (2015). The structure of plant photosystem I super-complex at 2.8 angstrom resolution. eLife, 4, 18.
Jordan, P., Fromme, P., Witt, H. T., Klukas, O., Saenger, W., & Krauss, N. (2001). Three-dimensional strucutre of cyanobacterial photosystem I at 2.5 A resolution. Nature, 411, 909–917.
Busch, A., & Hippler, M. (2011). The structure and function of eukaryotic photosystem I. Biochim. Biophys. Acta-Bioenerg., 1807, 864–877.
Jensen, P. E., Bassi, R., Boekema, E. J., Dekker, J. P., Jansson, S., Leister, D., Robinson, C., & Scheller, H. V. (2007). Structure, function and regulation of plant photosystem I. Biochim. Biophys. Acta-Bioenerg., 1767, 335–352.
Grotjohann, I., & Fromme, P. (2005). Structure of cyanobacterial photosystem I. Photosynthesis Research, 85, 51–72.
Nelson, N. (2008). Plant photosystem I—the most efficient nano-photochemical machine. Journal of Nanoscience and Nanotechnology, 8, 1–5.
Golbeck, J. H. (1992). Structure and function of photosystem I. Annual Review of Plant Physiology and Plant Molecular Biology, 43, 293–324.
Lubitz, W. (2006). In J. H. Golbeck (Ed.), Photosystem I: the light-driven plastocyanin: ferredoxin oxidoreductase (Vol. 24, pp. 246–269). Dordrecht: Springer.
Chitnis, P. R., Purvis, D., & Nelson, N. (1991). Molecular cloning and targeted mutagenesis of the gene psaF endcoding subunit III of photosystem I from the cyanobacterium Synechocystis sp. PCC 6803. The Journal of Biological Chemistry, 266, 20146–20151.
Fromme, P., Schubert, W.-D., & Krauss, N. (1994). Structure of photosystem I: suggestions on the docking sites for plastocyanin, ferredoxin and the coordination of P700. Biochimica et Biophysica Acta, 1187, 99–105.
Hippler, M., Reichert, J., Sutter, M., Zak, E., Altschmied, L., Schroer, U., Herrmann, R. G., & Haehnel, W. (1996). The plastocyanin binding domain of photosystem I. The EMBO Journal, 15, 6374–6384.
Semenov, A. Y., Petrova, A. A., Mamedov, M. D., & Nadtochenko, V. A. (2015). Electron transfer in photosystem I containing native and modified quinone acceptors. Biochemistry (Moscow), 80, 654–661.
Humphrey, W., Dalke, A., & Schulten, K. (1996). VMD—visual molecular dynamics. J. Molec. Graphics, 14, 33–38.
Zilber, A. L., & Malkin, R. (1992). Organization and topology of photosystem I subunits. Plant Physiology, 99, 901–911.
Lelong, C., Boekema, E. J., Kruip, J., Bottin, H., Rogner, M., & Setif, P. (1996). Characterization of a redox active corss-linked complex between cyanobacterial photosystem I and souble ferredoxin. The EMBO Journal, 15, 2160–2168.
Fritsch, J., Scheerer, P., Frielingsdorf, S., Kroschinsky, S., Friedrich, B., Lenz, O., & Spahn, C. M. T. (2011). The crystal structure of an oxygen-tolerant hydrogenase uncovers a novel iron-sulphur centre. Nature, 479, 249–253.
Volbeda, A., Charon, M.-H., Piras, C., Hatchikian, E. C., Frey, M., & Fontecilla-Camps, J. C. (1995). Crystal structure of the nickel-iron hydrogenase from Desulfovibrio gigas. Nature, 373, 580–587.
Peters, J. W., Lanzilotta, W. N., Lemon, B. J., & Seefeldt, L. C. (1998). X-ray crystal structure of the Fe-only hydrogenase (Cpl) from Clostridium pasteurianum to 1.8 angstrom resolution. Science, 282, 1853–1858.
Zirngibl, C., Vandongen, W., Schworer, B., Vonbunau, R., Richter, M., Klein, A., & Thauer, R. K. (1992). H2-forming methylenetetrahydromethanopterin dehydrognease, a novel type of hydrogenase without iron-sulfur clusters in methanogenic archaea. European Journal of Biochemistry, 208, 511–520.
Vignais, P., & Billoud, B. (2007). Occurrence, classification, and biological function of hydrogenases: an overview. Chemical Reviews, 107, 4206–4272.
Frey, M. (2002). Hydrogenases: hydrogen-activating enzymes. Chembiochem, 3, 153–160.
Goldet, G., Brandmayr, C., Stripp, S. T., Happe, T., Cavazza, C., Fontecilla-Camps, J. C., & Armstrong, F. A. (2009). Electrochemical kinetic investigations of the reactions of [FeFe]-hydrogenases with carbon monoxide and oxygen: comparing the importance of gas tunnels and active-site electronic/redox effects. Journal of the American Chemical Society, 131, 14979–14989.
Vincent, K. A., Parkin, A., Lenz, O., Albracht, S. P. J., Fontecilla-Camps, J. C., Cammack, R., Friedrich, B., & Armstrong, F. A. (2005). Electrochemical definitions of O2 sensitivity and oxidative inactivation in hydrogenases. Journal of the American Chemical Society, 127, 18179–18189.
Koo, J., Shiigi, S., Rohovie, M., Mehta, K., & Swartz, J. R. (2016). Characterization of [FeFe] hydrogenase O2 sensitivity using a new, physiological approach. The Journal of Biological Chemistry, 291, 21563–21570.
Goldet, G., Wait, A. F., Cracknell, J. A., Vincent, K. A., Ludwig, M., Lenz, O., Friedrich, B., & Armstrong, F. A. (2008). Hydrogen production under aerobic conditions by membrane-bound hydrogenases from Ralstonia species. Journal of the American Chemical Society, 130, 11106–11113.
Burgdorf, T., De Lacey, A. L., & Friedrich, B. (2002). Functional analysis by site-directed mutagenesis of the NAD(+)-reducing hydrogenase from Ralstonia eutropha. Journal of Bacteriology, 184, 6280–6288.
Reisner, E., Powell, D. J., Cavazza, C., Fontecilla-Camps, J. C., & Armstrong, F. A. (2009). Visible light-driven H2 production by hydrogenases attached to dye-sensitized TiO2 nanoparticles. Journal of the American Chemical Society, 131, 18457–18466.
Kanai, T., Ito, S., & Imanaka, T. (2003). Characterization of a cytosolic NiFe-hydrogenase from the hyperthermophilic archaeon Thermococcus kodakaraensis KOD1. Journal of Bacteriology, 185, 1705–1711.
Ma, K. S., Weiss, R., & Adams, M. W. W. (2000). Characterization of hydrogenase II from the hyperthermophilic archaeon Pyrococcus furiosus and assessment of its role in sulfur reduction. Journal of Bacteriology, 182, 1864–1871.
Maness, P.-C., Smolinski, S., Dillon, A. C., Heben, M. J., & Weaver, P. F. (2002). Characterization of the oxygen tolerance of a hydrogenase linked to a carbon monoxide oxidation pathway in Rubrivivax gelatinosus. Appl. Environ. Microb., 68, 2633–2636.
Schnackenberg, J., Miyake, M., Miyake, J., Zorin, N. A., & Asada, Y. (1999). In vitro and in vivo coupling of Thiocapsa hydrogenase with cyanobacterial and algal electron mediators. Journal of Bioscience and Bioengineering, 88, 30–34.
Cournac, L., Guedeney, G., Peltier, G., & Vignais, P. M. (2004). Sustained photoevolution of molecular hydrogen in a mutant of Synechocystis sp strain PCC 6803 deficient in the type I NADPH-dehydrogenase complex. Journal of Bacteriology, 186, 1737–1746.
Peters, J. W., Schut, G. J., Boyd, E. S., Mulder, D. W., Shepard, E. M., Broderick, J. B., King, P. W., & Adams, M. W. (2015). [FeFe]- and [NiFe]-hydrogenase diversity, mechanism, and maturation. Biochimica et Biophysica Acta, 1853, 1350–1369.
Ihara, M., Nakamoto, H., Kamachi, T., Okura, I., & Maeda, M. (2006). Photoinduced hydrogen production by direct electron transfer from photosystem I cross-linked with cytochrome c3 to [NiFe]-hydrogenase. Photochemistry and Photobiology, 82, 1677–1685.
Ihara, M., Nishihara, H., Yoon, K. S., Lenz, O., Friedrich, B., Nakamoto, H., Kojima, K., Honma, D., Kamachi, T., & Okura, I. (2006). Light-driven hydrogen production by a hybrid complex of a [NiFe]-hydrogenase and the cyanobacterial photosystem I. Photochemistry and Photobiology, 82, 676–682.
Krassen, H., Schwarze, A., Friedrich, B., Ataka, K., Lenz, O., & Heberle, J. (2009). Photosynthetic hydrogen production by a hybrid complex of photosystem I and [NiFe]-hydrogenase. ACS Nano, 3, 4055–4061.
Lubner, C. E., Applegate, A. M., Knorzer, P., Ganago, A., Bryant, D. A., Happe, T., & Golbeck, J. H. (2011). Solar hydrogen-producing bionanodevice outperforms natural photosynthesis. Proceedings of the National Academy of Sciences of the United States of America, 108, 20988–20991.
Lubner, C. E., Knorzer, P., Silva, P. J., Vincent, K. A., Happe, T., Bryant, D. A., & Golbeck, J. H. (2010). Wiring an [FeFe]-hydrogenase with photosystem I for light-induced hydrogen production. Biochemistry, 49, 10264–10266.
Mulder, D. W., Shepard, E. M., Meuser, J. E., Joshi, N., King, P. W., Posewitz, M. C., Broderick, J. B., & Peters, J. W. (2011). Insights into [FeFe]-hydrogenase structure, mechanism, and maturation. Structure, 19, 1038–1052.
Ogata, H., Lubitz, W., & Higuchi, Y. (2016). Structure and function of [NiFe] hydrogenases. Journal of Biochemistry, 160, 251–258.
Gaffron, H. (1939). Reduction of carbon dioxide with molecular hydrogen in green algae. Nature, 143, 204–205.
Gaffron, H., & Rubin, J. (1942). Fermentative and photochemical production of hydrogen in algae. The Journal of General Physiology, 26, 219–240.
Greenbaum, E. (1988). Energetic efficiency of hydrogen photoevolution by algal water splitting. Biophysical Journal, 54, 365–368.
Bayro-Kaiser, V., & Nelson, N. (2016). Temperature-sensitive PSII: a novel approach for sustained photosynthetic hydrogen production. Photosynthesis Research, 130, 113–121.
Kosourov, S., Tsygankov, A., Seibert, M., & Ghirardi, M. L. (2002). Sustained hydrogen photoproduction by Chlamydomonas reinhardtii: effects of culture parameters. Biotechnology and Bioengineering, 78, 731–740.
Melis, A., Zhang, L., Forestier, M., Ghirardi, M. L., & Seibert, M. (2000). Sustained photobiological hydrogen gas production upon reversible inactivation of oxygen evolution in the green alga Chlamydomonas reinhardtii. Plant Physiology, 122, 127–135.
Meuser, J. E., Ananyev, G., Wittig, L. E., Kosourov, S., Ghirardi, M. L., Seibert, M., Dismukes, G. C., & Posewitz, M. C. (2009). Phenotypic diversity of hydrogen production in chlorophycean algae reflects distinct anaerobic metabolisms. Journal of Biotechnology, 142, 21–30.
Pinto, T. S., Malcata, F. X., Arrabaca, J. D., Silva, J. M., Spreitzer, R. J., & Esquivel, M. G. (2013). Rubisco mutants of Chlamydomonas reinhardtii enhance photosynthetic hydrogen production. Applied Microbiology and Biotechnology, 97, 5636–5643.
Tolstygina, I. V., Antal, T. K., Kosourov, S. N., Krendeleva, T. E., Rubin, A. B., & Tsygankov, A. A. (2009). Hydrogen production by photoautotrophic sulfur-deprived Chlamydomonas reinhardtii pre-grown and incubated under high light. Biotechnology and Bioengineering, 102, 1055–1061.
Safari, F., Norouzi, O., & Tavasoli, A. (2016). Hydrothermal gasification of Cladophora glomerata macroalgae over its hydrochar as a catalyst for hydrogen-rich gas production. Bioresource Technology, 222, 232–241.
Happe, T., & Naber, J. D. (1993). Isolation, characterization and N-terminal amino acid sequence of hydrogenase from the green alga Chlamydomonas reinhardtii. Eur. J. of Biochem., 214, 475–481.
Schnackenberg, J., Schulz, R., & Senger, H. (1993). Characterization and purification of a hydrogenase from the eukaryotic green alga Scenedesmus obliquus. FEBS Letters, 327, 21–24.
Benemann, J. R., Berenson, J. A., Kaplan, N. O., & Kamen, M. D. (1973). Hydrogen evolution by a chloroplast-ferredoxin-hydrogenase system. Proceedings of the National Academy of Sciences of the United States of America, 70, 2317–2320.
Greenbaum, E. (1985). Platinized chloroplasts: a novel photocatalytic material. Science, 230, 1373.
Hall, D. O., Adams, M. W. W., Morris, P., & Rao, K. K. (1980). Photolysis of water for H2 production with the use of biological and artificial catalysts. Philos. T. Roy. Soc. A, 295, 473–476.
Rosen, M. M., & Krasna, A. I. (1980). Limiting reactions in hydrogen photoproduction by chloroplasts and hydrogenase. Photochemistry and Photobiology, 31, 259–265.
McTavish, H. (1997). Hydrogen evolution by direct electron transfer from photosystem I to hydrogenases. Journal of Biochemistry, 123, 644–649.
Grimme, R. A., Lubner, C. E., Bryant, D. A., & Golbeck, J. H. (2008). Photosystem I/molecular wire/metal nanoparticle bioconjugates for the photocatalytic production of H2. Journal of the American Chemical Society, 130, 6308–6309.
Grimme, R. A., Lubner, C. E., & Golbeck, J. H. (2009). Maximizing H2 production in photosystem I/dithiol molecular wire/platinum nanoparticle bioconjugates. Dalton Transactions, 10106–10113.
Benemann, J. R., & Weare, N. M. (1974). Hydrogen evolution by nitrogen-fixing Anabaena cylindrica cultures. Science, 184, 174–175.
Jeffries, T. W., Timourian, H., & Ward, R. L. (1978). Hydrogen production by Anabaena cylindrica: effects of varying ammonium and ferric ions, pH, and light. Appl. Environ. Microb., 35, 704–710.
Tsygankov, A. A., Fedorov, A. S., Kosourov, S. N., & Rao, K. K. (2002). Hydrogen production by cyanobacteria in an automated outdoor photobioreactor under aerobic conditions. Biotechnology and Bioengineering, 80, 777–783.
Harris, B. J., Cheng, X., & Frymier, P. (2016). Structure and function of photosystem I-[FeFe] hydrogenase protein fusion: an all-atom molecular dynamics study. The Journal of Physical Chemistry. B, 120, 599–609.
Gorka, M., Schartner, J., van der Est, A., Rogner, M., & Golbeck, J. H. (2014). Light-mediated hydrogen generation in photosystem I: attachment of a naphthoquinone-molecular wire-Pt nanoparticle to the A1A and A1B sites. Biochemistry, 53, 2295–2306.
Terasaki, N., Yamamoto, N., Hiraga, T., Yamanoi, Y., Yonezawa, T., Nishihara, H., Ohmori, T., Sakai, M., Fujii, M., Tohri, A., Iwai, M., Inoue, Y., Yoneyama, S., Minakata, M., & Enami, I. (2009). Plugging a molecular wire into photosystem I: reconstitution of the photoelectric conversion system on a gold electrode. Angewandte Chemie (International Ed. in English), 48, 1585–1587.
Le, R. K., Raeeszadeh-Sarmazdeh, M., Boder, E. T., & Frymier, P. D. (2015). Sortase-mediated ligation of PsaE-modified photosystem I from Synechocystis sp. PCC 6803 to a conductive surface for enhanced photocurrent production on a gold electrode. Langmuir, 31, 1180–1188.
Parthasarathy, R., Subramanian, S., & Boder, E. T. (2007). Sortase A as a novel molecular “stapler” for sequence-specific protein conjugation. Bioconjugate Chemistry, 18, 469–476.
Levary, D. A., Parthasarathy, R., Boder, E. T., & Ackerman, M. E. (2011). Protein-protein fusion catalyzed by sortase A. PloS One, 6, 1–6.
LeBlanc, G., Chen, G. P., Gizzie, E. A., Jennings, G. K., & Cliffel, D. E. (2012). Enhanced photocurrents of photosystem I films on p-doped silicon. Advanced Materials, 24, 5959.
Iwuchukwu, I. J., Iwuchukwu, E., Le, R., Paquet, C., Sawhney, R., Bruce, B., & Frymier, P. (2011). Optimization of photosynthetic hydrogen yield from platinized photosystem I complexes using response surface methodology. International Journal of Hydrogen Energy, 36, 11684–11692.
Acknowledgements
Funding for Baker Martin was provided by a fellowship from the GAANN program of the US Department of Education, Grant Number P200A150281.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflicts of interest.
Electronic supplementary material
ESM 1
(DOCX 21 kb)
Rights and permissions
About this article
Cite this article
Martin, B.A., Frymier, P.D. A Review of Hydrogen Production by Photosynthetic Organisms Using Whole-Cell and Cell-Free Systems. Appl Biochem Biotechnol 183, 503–519 (2017). https://doi.org/10.1007/s12010-017-2576-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-017-2576-3