Abstract
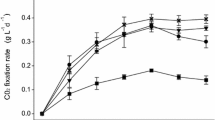
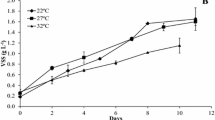
In the present study, the capacity of the cyanobacterium Leptolyngbya sp. CChF1 to remove CO2 from real and synthetic biogas was evaluated. The identification of the cyanobacterium, isolated from the lake Chapala, was carried out by means of morphological and molecular analyses, while its potential for CO2 removal from biogas streams was evaluated by kinetic experiments and optimized by a central composite design coupled to a response surface methodology. Results demonstrated that Leptolyngbya sp. CChF1 is able to remove CO2 and grow indistinctly in real or synthetic biogas streams, showing tolerance to high concentrations of CO2 and CH4, 25 and 75%, respectively. The characterization of the biomass composition at the end of the kinetic assays revealed that the main accumulated by-products under both biogas streams were lipids, followed by proteins and carbohydrates. Regarding the optimization experiments, light intensity and temperature were the studied variables, while synthetic biogas was the carbon source. Results showed that light intensity was significant for CO2 capture efficiency (p = 0.0290), while temperature was significant for biomass production (p = 0.0024). The predicted CO2 capture efficiency under optimal conditions (27.1 °C and 920 lx) was 93.48%. Overall, the results of the present study suggest that Leptolyngbya sp. CChF1 is a suitable candidate for biogas upgrading.






Similar content being viewed by others
References
Ghorbani, A., Rahimpour, H. R., Ghasemi, Y., Zoughi, S., & Rahimpour, M. R. (2014). A review of carbon capture and sequestration in Iran: microalgal biofixation potential in Iran. Renewable and Sustainable Energy Reviews, 35, 73–100.
Meier, L., Pérez, R., Azócar, L., Rivas, M., & Jeison, D. (2015). Photosynthetic CO2 uptake by microalgae: an attractive tool for biogas upgrading. Biomass and Bioenergy, 73, 102–109.
Kao, C. Y., Chiu, S. Y., Huang, T. T., Dai, L., Wang, G. H., Tseng, C. P., Chen, C. H., & Lin, C. S. (2012). A mutant strain of microalga Chlorella sp. for the carbon dioxide capture from biogas. Biomass and Bioenergy, 36, 132–140.
Yan, C., & Zheng, Z. (2013). Performance of photoperiod and light intensity on biogas upgrade and biogas effluent nutrient reduction by the microalgae Chlorella sp. Bioresource Technology, 139, 292–299.
Yan, C., & Zheng, Z. (2014). Performance of mixed LED light wavelengths on biogas upgrade and biogas fluid removal by microalga Chlorella sp. Applied Energy, 113, 1008–1014.
Rosgaard, L., de Porcellinis, A. J., Jacobsen, J. H., Frigaard, N. U., & Sakuragi, Y. (2012). Bioengineering of carbon fixation, biofuels, and biochemicals in cyanobacteria and plants. Journal of Biotechnology, 162, 134–147.
Machado, I. M. P., & Atsumi, S. (2012). Cyanobacterial biofuel production. Journal of Biotechnology, 162, 50–56.
Zeng, X., Guo, X., Su, G., Danquah, M. K., Zhang, S., Lu, Y., Sun, Y., & Lin, L. (2015). Bioprocess considerations for microalgal-based wastewater treatment and biomass production. Renewable and Sustainable Energy Reviews, 42, 1385–1392.
Thiansathit, W., Keener, T. C., Khang, S. J., Ratpukdi, T., & Hovichitr, P. (2015). The kinetics of Scenedesmus obliquus microalgae growth utilizing carbon dioxide gas from biogas. Biomass and Bioenergy, 76, 79–85.
Mendez, L., Mahdy, A., Ballesteros, M., & González-Fernández, C. (2015). Chlorella vulgaris Vs cyanobacterial biomasses: comparison in terms of biomass productivity and biogas yield. Energy Conversion and Management, 92, 137–142.
Ullah, K., Ahmad, M., Sofia, Sharma, V. K., Lu, P., Harvey, A., Zafar, M., & Sultana, S. (2015). Assessing the potential of algal biomass opportunities for bioenergy industry: A review. Fuel, 143, 414–423.
Bellou, S., Baeshen, M. N., Elazzazy, A. M., Aggeli, D., Sayegh, F., & Aggelis, G. (2014). Microalgal lipids biochemistry and biotechnological perspectives. Biotechnology Advances, 32, 1476–1493.
Kao, C. Y., Chen, T. Y., Chang, Y. B., Chiu, T. W., Lin, H. Y., Chen, C. D., Chang, J. S., & Lin, C. S. (2014). Utilization of carbon dioxide in industrial flue gases for the cultivation of microalga Chlorella sp. Bio/Technology, 166, 485–493.
Quintana, N., Van Der Kooy, F., Van De Rhee, M. D., Voshol, G. P., & Verpoorte, R. (2011). Renewable energy from cyanobacteria: energy production optimization by metabolic pathway engineering. Applied Microbiology and Biotechnology, 9, 471–490.
Singh, J., Tripathi, R., & Thakur, I. S. (2014). Characterization of endolithic cyanobacterial strain, Leptolyngbya sp. ISTCY101, for prospective recycling of CO2 and biodiesel production. Bioresource Technology, 166, 345–352.
Sarsekeyeva, F., Zayadan, B. K., Usserbaeva, A., Bedbenov, V. S., Sinetova, M. A., & Los, D. A. (2015). Cyanofuels: biofuels from cyanobacteria. Reality and perspectives. Photosynthesis Research, 125, 329–340.
Valério, E., Chambel, L., Paulino, S., Faria, N., Pereira, P., & Tenreiro, R. (2009). Molecular identification, typing and traceability of cyanobacteria from freshwater reservoirs. Microbiology, 155, 642–656.
Nübel, U., Garcia-pichel, F., & Muyzer, G. (1997). PCR primers to amplify 16S rRNA genes from cyanobacteria. Microbiology, 63, 3327–3332.
Hays, S. G., & Ducat, D. C. (2015). Engineering cyanobacteria as photosynthetic feedstock factories. Photosynthesis Research, 123, 285–295.
Kumar, K., Dasgupta, C. N., Nayak, B., Lindblad, P., & Das, D. (2011). Development of suitable photobioreactors for CO2 sequestration addressing global warming using green algae and cyanobacteria. Bioresource Technology, 102, 4945–4953.
Zhao, B., & Su, Y. (2014). Process effect of microalgal-carbon dioxide fixation and biomass production: a review. Renewable and Sustainable Energy Reviews, 31, 121–132.
Smith, D. L., Johnson, K. B., & Darmstadt, T. U. (1996). A guide to marine coastal plankton and marine invertebrate larvae (2nd ed.). Iowa: Kendall/Hunt Publishing Company.
Raoof, B., Kaushik, B. D., & Prasanna, R. (2006). Formulation of a low-cost medium for mass production of Spirulina. Biomass and Bioenergy, 30, 537–542.
Godon, J. J., Zumstein, E., Dabert, P., Habouzit, F., & Moletta, R. (1997). Molecular microbial diversity of an anaerobic digestor as determined by small-subunit rDNA sequence analysis. Apllied and Environmental Microbiology, 63, 2802–2813.
Saitou, N., & Nei, M. (1987). The neighbor-joining method: a new method for reconstructing phylogenetic trees. Molecular Biology and Evolution, 4, 406–425.
Arreola-Vargas, J., Ojeda-Castillo, V., Snell-Castro, R., Corona-González, R. I., Alatriste-Mondragón, F., & Méndez-Acosta, H. O. (2015). Methane production from acid hydrolysates of Agave tequilana bagasse: evaluation of hydrolysis conditions and methane yield. Bioresource Technology, 181, 191–199.
Tang, D., Han, W., Li, P., Miao, X., & Zhong, J. (2011). CO2 biofixation and fatty acid composition of Scenedesmus obliquus and Chlorella pyrenoidosa in response to different CO2 levels. Bioresource Technology, 102, 3071–3076.
Chisti, Y. (2007). Biodiesel from microalgae. Biotechnology Advances, 25, 294–306.
Kao, C. Y., Chiu, S. Y., Huang, T. T., Dai, L., Hsu, L. K., & Lin, C. S. (2012b). Ability of a mutant strain of the microalga Chlorella sp. to capture carbon dioxide for biogas upgrading. Applied Energy, 93, 176–183.
Dubois, M., Gilles, K. A., Hamilton, J. K., Rebers, P. A., & Smith, F. (1956). Colorimetric method for determination of sugars and related substances. Analytical Chemistry, 28, 350–356.
Lowry, O. H., Rosebrough, N. J., Farr, A. L., & Randall, R. J. (1951). Protein measurement with the Folin phenol reagent. The Journal of Biological Chemistry, 193, 265–275.
Bligh, E. G., & Dyer, W. J. (1959). A rapid method of total lipid extraction and purification. Canadian Journal of Biochemistry and Physiology, 37, 911–917.
Wehr, J. D., Sheath, R. G., & Kociolek, J. P. (2015). Freshwater algae of North America: ecology and classification (2nd ed.). San Diego: Elsevier.
Castiglioni, B., Rizzi, E., Frosini, A., Sivonen, K., Rajaniemi, P., Rantala, A., Mugnai, M. A., Ventura, S., Wilmotte, A., Boutte, C., Grubisic, S., Balthasart, P., Consolandi, C., Bordoni, R., MezzelanI, A., Battaglia, C., & De Bellis, G. (2004). Development of a universal microarray based on the ligation detection reaction and 16S rRNA gene polymorphism to target diversity of cyanobacteria. Applied and Environmental Microbiology, 70, 7161–7172.
de Araujo, E. D., Patel, J., de Araujo, C., Rogers, S. P., Short, S. M., Campbell, D. A., & Espie, G. S. (2011). Physiological characterization and light response of the CO2-concentrating mechanism in the filamentous cyanobacterium Leptolyngbya sp. CPCC 696. Photosynthesis Research, 109, 85–101.
Burnap, R. L., Hagemann, M., & Kaplan, A. (2015). Regulation of CO2 concentrating mechanism in cyanobacteria. Life, 5, 348–371.
Solovchenko, A., & Khozin-Goldberg, I. (2013). High-CO2 tolerance in microalgae: possible mechanisms and implications for biotechnology and bioremediation. Biotechnology Letters, 35, 1745–1752.
Bahr, M., Díaz, I., Dominguez, A., González Sachez, A., & Muñoz, R. (2014). Microalgal-biotechnology as a platform for an integral biogas upgrading and nutrient removal from anaerobic effluents. Environmental Science and Technology, 48, 573–581.
Posadas, E., Serejo, M. L., Blanco, S., Pérez, R., García-Encina, P. A., & Muñoz, R. (2015). Minimization of biomethane oxygen concentration during biogas upgrading in algal-bacterial photobioreactors. Algal Research, 12, 221–229.
Converti, A., Oliveira, R. P. S., Torres, B. R., Lodi, A., & Zilli, M. (2009). Biogas production and valorization by means of a two-step biological process. Bioresource Technology, 100, 5771–5776.
de Morais, M. G., & Costa, J. A. V. (2007). Biofixation of carbon dioxide by Spirulina sp. and Scenedesmus obliquus cultivated in a three-stage serial tubular photobioreactor. Journal of Biotechnology, 129, 439–445.
Choix, F. J., de- Bashan, L. E., & Bashan, Y. (2012). Enhanced accumulation of starch and total carbohydrates in alginate-immobilized Chlorella spp. induced by Azospirillum brasilense: II. Heterotrophic conditions. Enzyme and Microbial Technology, 51(5), 300–309.
Takano, H., Takeyama, H., Nakamura, N., Sode, K., Burgess, J. G., Manabe, E., et al. (1992). CO2 removal by high-density culture of a marine cyanobacterium Synechococcus sp. using an improved photobioreactor employing light-diffusing optical fibers. Applied Biochemistry and Biotechnology, 34, 449–458.
Li, F. F., Yang, Z. H., Zeng, R., Yang, G., Chang, X., Yan, J. B., & Hou, Y. L. (2011). Microalgae capture of CO2 from actual flue gas discharged from a combustion chamber. Industrial & Engineering Chemistry Research, 50, 6496–6502.
González-Fernández, C., & Ballesteros, M. (2012). Linking microalgae and cyanobacteria culture conditions and key-enzymes for carbohydrate accumulation. Biotechnology Advances, 30, 1655–1661.
Tongprawhan, W., Srinuanpan, S., & Cheirsilp, B. (2014). Biocapture of CO2 from biogas by oleaginous microalgae for improving methane content and simultaneously producing lipid. Bioresource Technology, 170, 90–99.
Acknowledgments
We thank the Fondo Sectorial CONACYT-SENER-Sustentabilidad Energética, Clúster Biocombustibles Gaseosos project 247006 for financial support. Francisco J. Choix acknowledges CONACYT for the support under the Cátedras CONACYT project 2517. Finally, the technical support provided by Dra. Rocio Gonzalez-Castañeda and Dr. Fernando Jáuregui-Huerta from the Neurosciences laboratory (microscopy) of CUCS—Universidad de Guadalajara is also acknowledged.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Choix, F.J., Snell-Castro, R., Arreola-Vargas, J. et al. CO2 Removal from Biogas by Cyanobacterium Leptolyngbya sp. CChF1 Isolated from the Lake Chapala, Mexico: Optimization of the Temperature and Light Intensity. Appl Biochem Biotechnol 183, 1304–1322 (2017). https://doi.org/10.1007/s12010-017-2499-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-017-2499-z