Abstract
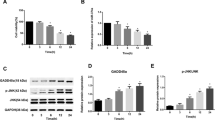
Isoliquiritigenin (ISL) has been reported to have a wide range of biological activities. This study evaluated the cytotoxic effect of ISL on norvegicus pheochromocytoma cell line (PC-12 cells) and its possible molecular mechanism. The cytotoxicity in vitro of ISL against PC-12 cells was investigated by MTT assay. The migration and invasion of PC-12 cells were performed by scratch test and transwell assay. Apoptosis was evaluated by microscopy and flow cytometry. The reactive oxygen species (ROS) and mitochondrial membrane potential were studied by fluorescent microscopy. DNA damage of PC-12 cells was analyzed by comet assay. The protein expression of caspase, Bcl-2 family member, autophagy-associated protein Beclin-1, and LC3 was detected by western blot. The autophagy of PC-12 cells was investigated by acridine orange (AO) and monodansylcadaverine (MDC) staining. The IC50 value of ISL against PC-12 cell is 17.8 ± 1.8 μM. ISL could suppress PC-12 cell migration and invasion. AO/ethidium bromide staining and flow cytometry suggested that ISL caused apoptosis of PC-12 cells. Significant DNA damages of PC-12 cells treated with ISL were observed in a comet assay. ISL inhibited the cell growth of PC-12 cells at S phase. Exposure of PC-12 cells to ISL increased the levels of cellular reactive oxygen species (ROS) and decreased the mitochondrial membrane potential. Additionally, ISL trigged the release of cytochrome c from the mitochondria to the cytoplasm. The expression levels of caspase-9, caspase-3, caspase-7, Bax, and Bim were upregulated, whereas the expression levels of Bcl-2 and Bcl-x were downregulated. AO and monodansylcadaverine (MDC) staining assay showed that ISL caused autophagy of PC-12 cells. The upregulation of protein Beclin-1 and LC3 was observed in PC-12 cells. Therefore, the results show that ISL induces apoptosis of PC-12 cells through ROS-mediated activation of the intrinsic mitochondria-cytochrome c-caspase protease mechanism and causes the autophagy of PC-12 cells.

The in vitro cytotoxicity, apoptosis, comet assay, ROS, mitochondrial membrane potential, cell cycle arrest, autophagy, and western blot induced by ISL were investigated.












Similar content being viewed by others
References
Evan, G. I., & Vousden, K. H. (2001). Proliferation cell cycle and apoptosis in cancer. Nature, 41, 342–348.
Fulda, S., & Debatin, K. M. (2004). Signaling through death receptors in cancer therapy. Current Opinion in Pharmacology, 4, 327–332.
Debatin, K. M., & Krammer, P. H. (2004). Death receptors in chemotherapy and cancer. Oncogene, 23, 2950–2966.
Hengartner, M. O. (2000). The biochemistry of apoptosis. Nature, 40, 770–776.
Kim, H. E., Du, F., Fang, M., & Wang, X. (2005). Formation of apoptosome is initiated by cytochrome c-induced dATP hydrolysis and subsequent nucleotide exchange on Apaf-1. Proceedings of the National Academy of Sciences of the United States of America, 102, 17545–17550.
Fang, H. Q., Wu, Y. L., Guo, J. B., Rong, J., Ma, L., Zhao, Z. M., Zuo, D. Y., & Peng, S. Q. (2012). T-2 toxin induces apoptosis in differentiated murine embryonic stem cells through reactive oxygen species-mediated mitochondrial pathway. Apoptosis, 17, 895–907.
Song, W., Li, S. S., Qiu, P. P., Shen, D. Y., Tian, L., Zhang, Q. Y., Liao, L. X., & Chen, Q. X. (2013). Apoptosis induced by aqueous extracts of crocodile bile in human heptacarcinoma SMMC-7721. Applied Biochemistry and Biotechnology, 170, 15–24.
Wakx, A., Dutot, M., Massicot, F., Mascarelli, G. F., Limb, A., & Rat, P. (2016). Amyloid β peptide induces apoptosis through P2X7 cell death receptor in retinal cells: modulation by marine omega-3 fatty acid DHA and EPA. Applied Biochemistry and Biotechnology, 178, 368–381.
Hotchkiss, R. S., Strasser, A., Mc Dunn, J. E., & Swanson, P. E. (2009). Cell death. The New England Journal of Medicine, 361, 1570–1583.
Tan, C. P., Lai, S. S., Wu, S. H., Hu, S., & Xu, A. L. (2010). Nuclear permeable ruthenium(II) β-carboline complexesinduce autophagyto antagonize mitochondrial mediated apoptosis. Journal of Medicinal Chemistry, 53, 7613–7624.
Maycotte, P., & Thorburn, A. (2011). Autophagy and cancer therapy. Cancer Biology & Therapy, 11, 127–137.
Yang, C., Kaushal, V., Shah, S. V., & Kaushal, G. P. (2008). Autophagy is associated with apoptosis in cisplatin injury to renal tubular epithelial cells. American Journal of Physiology Renal, Fluid Electrolyte Physiology, 294, 777–787.
Li, X., Xu, H. L., Liu, Y. X., An, N., Zhao, S., & Bao, J. K. (2013). Autophagy modulation as a target for anticancer drug discovery. Acta Pharmacologica Sinica, 34, 612–624.
Yuan, L., Wei, S. P., Wang, J., & Liu, X. B. (2014). Isoorientin induces apoptosis and autophagy simultaneously by reactive oxygen species (ROS)-related p53, PI3K/Akt, JNK, and p38 signaling pathways in HepG2 cancer cells. Journal of Agricultural and Food Chemistry, 62, 5390–5400.
Chen, G., Zhu, L., Liu, Y., Zhou, Q., Chen, H., & Yang, J. (2009). Isoliquiritigenin, a flavonoid from licorice, plays a dual role in regulating gastrointestinal motility in vitro and in vivo. Phytotherapy Research, 23, 498–506.
Sun, Z. J., Chen, G., Zhang, W., Hu, X., Huang, C. F., Wang, Y. F., Jia, J., & Zhao, Y. F. (2010). Mammalian target of rapamycin pathway promotes tumor-induced angiogenesis in adenoid cystic carcinoma: its suppression by isoliquiritigenin through dual activation of c-Jun NH2-terminal kinase and inhibition of extracellular signalregulated kinase. The Journal of Pharmacology and Experimental Therapeutics, 334, 500–512.
Park, S. J., Song, H. Y., & Youn, H. S. (2009). Suppression of the TRIF-dependent signaling pathway of toll-like receptors by isoliquiritigenin in RAW264.7 macrophages. Molecular Cell, 28, 365–368.
Iwashita, K., Kobori, M., Yamaki, K., & Tsushida, T. (2000). Flavonoids inhibit cell growth and induce apoptosis in B16 melanoma 4A5 cells. Bioscience, Biotechnology, and Biochemistry, 64, 1813–1820.
Chen, X., Yang, M., Hao, W., Han, J., Ma, J., Wang, C., Sun, S., & Zheng, Q. (2016). Differentiation-inducing and anti-proliferative activities of isoliquiritigenin and all-trans-retinoic acid on B16F0 melanoma cells: mechanisms profiling by RNA-seq. Gene, 592, 86–89.
Wang, Y., Ma, J., Yan, X., Chen, X., Si, L., Liu, Y., Han, J., Hao, W., & Zheng, Q. (2016). Isoliquiritigenin inhibits proliferation and induces apoptosis via alleviating hypoxia and reducing glycolysis in mouse melanoma B16F10 cells. Recent Patents on Anti-Cancer Drug Discovery, 11, 215–217.
Maggiolini, M., Statti, G., Vivacqua, A., Gabriele, S., Rago, V., Loizzo, M., Menichini, F., & Amdo, S. (2002). Estrogenic and antiproliferative activities of isoliquiritigenin in MCF7 breast cancer cells. The Journal of Steroid Biochemistry and Molecular Biology, 82, 315–322.
Hsu, Y. L., Kuo, P. L., & Lin, C. C. (2005). Isoliquiritigenin induces apoptosis and cell cycle arrest through p53-dependent pathway in HepG2 cells. Life Sciences, 77, 279–292.
Hsu, Y. L., Kuo, P. L., Lin, L. T., & Lin, C. C. (2005). Isoliquiritigenin inhibits cell proliferation and induces apoptosis in human hepatoma cells. Planta Medica, 71, 130–134.
Jung, J. I., Chung, E., Seon, M. R., Shin, H. K., Kim, E. J., Lim, S. S., Chung, W. Y., Park, K. K., & Park, J. H. Y. (2006). Isoliquiritigenin (ISL) inhibits ErbB3 signaling in prostate cancer cells. BioFactors, 28, 159–168.
Jung, J. I., Lim, S. S., Choi, H. J., Cho, H. J., Shin, H. K., Kim, E. J., Chung, W. Y., Park, K. K., & Park, J. H. Y. (2006). Isoliquiritigen ininduces apoptosis by depolarizing mitochondrial membranes in prostate cancer cells. The Journal of Nutritional Biochemistry, 17, 689–696.
Zhang, X. Y., Yeung, E. D., Wang, J. Y., Panzhinskiy, E. E., Tong, C., Li, W. G., & Li, J. (2010). Isoliquiritigenin a natural anti-oxidant selectively inhibits the proliferation ofprostate cancer cells. Clinical and Experiment Pharmacology & Physiology, l37, 841–847.
Li, D., Wang, Z., Chen, H., Wang, J., Zheng, Q., Shang, J., & Li, J. (2009). Isoliquiritigenin induces monocytic differentiation of HL-60 cells. Free Radical Biology & Medicine, 46, 731–736.
Ghobrial, I. M., Witzig, T. E., & Adjei, A. A. (2005). Targeting apoptosis pathways in cancertherapy. Cancer Journal for Clinicians, 55, 178–194.
Li, W., Jiang, G. B., Yao, J. H., Wang, X. Z., Wang, J., Han, B. J., Xie, Y. Y., Lin, G. J., Huang, H. L., & Liu, Y. J. (2014). Ruthenium(II) complexes: DNA-binding, cytotoxicity, apoptosis, cellular localization, cell cycle arrest, reactive oxygen species, mitochondrial membrane potential and western blot analysis. Journal of Photochemistry and Photobiology B: Biology, 14, 094–104.
Alapetite, C., Wachter, T., Sage, E., & Moustacchi, E. (1996). Use of the alkaline comet assay to detect DNA repair deficiencies in human fibroblasts exposed to UVC, UVB, UVA and gamma-rays. International Journal of Radiation Biology, 69, 359–369.
Silveira, L. R., Pereira-Da-Silva, L., Juel, C., & Hellsten, Y. (2003). Formation of hydrogen peroxide and nitric oxide in rat skeletal muscle cells during contractions. Free Radical Biology & Medicine, 35, 455–464.
Ito, K., Hirao, A., Arai, F., Takubo, K., Matsuoka, S., Miyamoto, K., Ohmura, M., Naka, K., Hosokawa, K., Ikeda, Y., & Suda, T. (2006). Reactive oxygen species act through p38 MAPK to limit the lifespan of hematopoietic stem cells. Nature Medicine, 12, 446–451.
Chen, H. M., Zhang, B., Yao, Y., Chen, N., Chen, X. Y., Tian, H., Wang, Z. H., & Zheng, Q. S. (2012). NADPH oxidase-derived reactive oxygen species are involved in the HL-60 cell monocytic differentiation induced by isoliquiritigenin. Molecules, 17, 13424–13438.
Yuan, X., Zhang, B., Chen, N., Chen, X. Y., Liu, L. L., Zheng, Q. S., & Wang, Z. P. (2012). Isoliquiritigenin treatment induces apoptosis by increasing intracellular ROS levels in HeLa cells. Journal of Asian Natural Products Research, 14, 789–798.
Thress, K., Kornbluth, S., & Smith, J. J. (1999). Mitochondria at the crossroad of apoptotic cell death. Bioenergetics Biomembranes, 31, 321–326.
Chen, H. M., Zhang, B., Yuan, X., Yao, Y., Zhao, H., Sun, X. L., & Zheng, Q. S. (2013). Isoliquiritigenin-induced effects on Nrf2 mediated antioxidant defence in the HL-60 cell monocytic differentiation. Cell Biology International, 37, 1215–1224.
Maiuri, M. C., Zalckvar, E., Kimchi, A., & Kroemer, G. (2007). Self-eating and self-killing: crosstalk between autophagy and apoptosis. Molecular and Cellular Biology, 8, 741–745.
Song, W., Yang, H. B., Chen, P., Wang, S. M., Zhao, L. P., Xu, W. H., Fan, H. F., Gu, X., & Chen, L. Y. (2013). Apoptosis of human gastric carcinoma SGC-7901 induced by deoxycholic acid via the mitochondrial-dependent pathway. Applied Biochemistry and Biotechnology, 171, 1061–1071.
Takahashi, T., Takasuka, N., Iigo, M., Baba, M., Nishino, H., Tsuda, H., & Okuyama, T. (2004). Isoliquiritigenin, a flavonoid from licorice, reduces prostaglandin E2 and nitric oxide, causes apoptosis, andsuppresses aberrant crypt foci development. Cancer Science, 95, 448–453.
Chen, G., Hu, X., Zhang, W., Xu, N., Wang, F. Q., Jia, J., Zhang, W. F., Sun, Z. J., & Zhao, Y. F. (2012). Mammalian target of rapamycin regulates isoliquiritigenin induced autophagic and apoptotic cell death in adenoid cystic carcinoma cells. Apoptosis, 17, 90–101.
Madamanchi, N. R., Moon, S. K., Hakim, Z. S., Clark, S., Mehrizi, A., Patterson, C., & Runge, M. S. (2005). Differential activation of mitogenic signaling pathways in aortic smooth muscle cells deficient in superoxide dismutase isoforms. Arteriosclerosis, Thrombosis, and Vascular Biology, 25, 950–956.
Poli, G. (2004). Oxidative stress and cell signalling. Current Medicinal Chemistry, 11, 1163–1182.
Yuan, X., Zhang, B., Gan, L., Wang, Z. H., Yu, B. C., Liu, L. L., Zheng, Q. S., & Wang, Z. P. (2013). Involvement of the mitochondrion-dependent and the endoplasmic reticulum stress-signaling pathways in isoliquiritigenin-induced apoptosis of HeLa cell. Biomedical and Environmental Sciences, 26, 268–276.
Singh, N. P. (1988). A simple technique for quantification of low levels of DNA damage in individual cells. Experimental Cell Research, 175, 184–191.
Orrenius, S., Gogvadze, V., & Zhivotovsky, B. (2007). Mitochondrial oxidative stress: implications for cell death. Annual Review of Pharmacology and Toxicology, 47, 143–183.
Li, P. (1997). Cytochrome c and dATP-dependent formation ofApaf-1/caspase-9 complex initiates an apoptotic protease cascade. Cell, 91, 479–489.
Kaufmann, S. H., & Hengartner, M. O. (2001). Programmed cell death: alive and well in the new millennium. Trends in Cell Biology, 11, 526–534.
Arthur, C. R., Gupton, J. T., Kellogg, G. E., Yeudall, W. A., Cabot, W. C., Newsham, I. F., & Gewirtz, D. A. (2007). Autophagic cell death, polyploidy and senescence induced inbreast tumor cells by the substituted pyrrole JG-03-14, a novelmicrotubule poison. Biochemical Pharmacology, 74, 981–991.
Acknowledgments
This work was supported by the Priority Academic Program Development of Guangdong Higher Education Institution (2013LYM0047), the Natural Science Foundation of Guangdong Province (No. 2016A030313728) and the project of innovation for enhancing Guangdong Pharmceutical University, provincial experimental teaching demonstration center of chemistry and chemical engineering.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Research Highlights
• The cytotoxicity of ISL was evaluated by MTT method.
• The apoptosis was investigated with AO/EB and flow cytometry.
• The cycle arrest, ROS, and mitochondrial membrane potential were studied.
• Investigation of the expression of proteins in apoptosis pathway was performed.
• The autophagy and autophagy-associated proteins were analyzed.
Rights and permissions
About this article
Cite this article
Yang, HH., Zhang, C., Lai, SH. et al. Isoliquiritigenin Induces Cytotoxicity in PC-12 Cells In Vitro. Appl Biochem Biotechnol 183, 1173–1190 (2017). https://doi.org/10.1007/s12010-017-2491-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-017-2491-7