Abstract
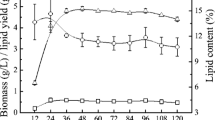
Cellulosic ethanol fermentation wastewater is the stillage stream of distillation column of cellulosic ethanol fermentation broth with high chemical oxygen demand (COD). The COD is required to reduce before the wastewater is released or recycled. Without any pretreatment nor external nutrients, the cellulosic ethanol fermentation wastewater bioconversion by Trichosporon cutaneum ACCC 20271 was carried out for the first time. The major components of the wastewater including glucose, xylose, acetic acid, ethanol, and partial of phenolic compounds could be utilized by T. cutaneum ACCC 20271. In a 3-L bioreactor, 2.16 g/L of microbial lipid accumulated with 55.05% of COD reduced after a 5-day culture of T. cutaneum ACCC 20271 in the wastewater. The fatty acid composition of the derived microbial lipid was similar with vegetable oil, in which it could be used as biodiesel production feedstock. This study will both solve the environmental problem and offer low-cost lipid feedstock for biodiesel production.


Similar content being viewed by others
References
Mosier, N., Wyman, C., Dale, B., Elander, R., Lee, Y. Y., Holtzapple, M., & Ladisch, M. (2005). Features of promising technologies for pretreatment of lignocellulosic biomass. Bioresource Technology., 96(6), 673–686.
Palmqvist, E., & Hahn-Hagerdal, B. (2000). Fermentation of lignocellulosic hydrolysates. II: inhibitors and mechanisms of inhibition. Bioresource Technology., 74(1), 25–33.
Humbird, D., Davis, R., Tao, L., Kinchin, C., Hsu, D., Aden, A. (2011). Process design and economics for biochemical conversion of lignocellulosic biomass to ethanol. Technical report. NREL/TP-5100-47764
Qureshi, A. S., Zhang, J., & Bao, J. (2015). High ethanol fermentation performance of the dry dilute acid pretreated corn stover by an evolutionarily adapted Saccharomyces cerevisiae strain. Bioresource Technology., 189, 399–404.
Sarkar, N., Ghosh, S. K., Bannerjee, S., & Aikat, K. (2012). Bioethanol production from agricultural wastes: an overview. Renewable Energy, 37(1), 19–27.
Zhao, Y. B. (2013). Process design of wastewater treatment for the NREL cellulosic ethanol model. Advanced Materials Research., 777, 365–369.
Wang, Z., & Zheng, W. (2012). Study on the treatment of wastewater from cellulose ethanol production and its engineering application. Industrial Water Treatment., 32(8), 88–91.
Liu, J, Zhang, C., Zhang, G., Gan, H. (2011). Reclaiming bioenergy from alcohol wastewater by upflow anaerobic solid reactor process and high value use of biogas. International Conference on New Technology of Agricultural Engineering, Zibo, China. 537–539
Gude, V. G. (2016). Wastewater treatment in microbial fuel cells—an overview. Journal of Cleaner Production., 122, 287–307.
Xue, F. Y., Zhang, X., Luo, H., & Tan, T. (2006). A new method for preparing raw material for biodiesel production. Process Biochemistry., 41(7), 1699–1702.
Hall, J., Hetrick, M., French, T., Hernandez, R., Donaldson, J., Mondala, A., & Holmes, W. (2011). Oil production by a consortium of oleaginous microorganisms grown on primary effluent wastewater. Journal of Chemical Technology and Biotechnology, 86(1), 54–60.
Ling, J., Nip, S., & Shim, H. (2013). Enhancement of lipid productivity of Rhodosporidium toruloides in distillery wastewater by increasing cell density. Bioresource Technology., 146C(10), 301–309.
Zhou, W., Wang, W., Li, Y., & Zhang, Y. (2013). Lipid production by Rhodosporidium toruloides Y2 in bioethanol wastewater and evaluation of biomass energetic yield. Bioresource Technology., 127(1), 435–440.
Peng, W., Huang, C., Chen, X., Xiong, L., Chen, X., Chen, Y., & Ma, L. (2013). Microbial conversion of wastewater from butanol fermentation to microbial oil by oleaginous yeast Trichosporon dermatis. Renewable Energy., 55(C), 31–34.
Chen, X., Li, Z., Zhang, X., Hu, F., Dewey, D. Y., & Bao, J. (2009). Screening of oleaginous yeast strains tolerant to lignocellulose degradation compounds. Applied Biochemistry and Biotechnology., 159(3), 591–604.
Huang, C., Chen, X. F., Xiong, L., Chen, X. D., Ma, L. L., & Chen, Y. (2013). Single cell oil production from low-cost substrates: the possibility and potential of its industrialization. Biotechnology Advances., 31(2), 129–139.
Papanikolaou, S., & Aggelis, G. (2011). Lipids of oleaginous yeasts. Part II: technology and potential applications. European Journal of Lipid Science and Technology., 113(8), 1052–1073.
Wang, J., Gao, Q., Zhang, H., & Bao, J. (2016). Inhibitor degradation and lipid accumulation potentials of oleaginous yeast Trichosporon cutaneum using lignocellulose feedstock. Bioresource Technology., 218, 892–901.
Hu, C., Wu, S., Wang, Q., Jin, G., Shen, H., & Zhao, Z. K. (2011). Simultaneous utilization of glucose and xylose for lipid production by Trichosporon cutaneum. Biotechnology for Biofuels., 4(1), 25.
Mörtberg, M., & Neujahr, H. Y. (1986). Transport and hydrolysis of disaccharides by Trichosporon cutaneum. Journal of Bacteriology., 168(2), 734–738.
Zhang, J., Zhu, Z., Wang, X., Wang, N., Wang, W., & Bao, J. (2010). Biodetoxification of toxins generated from lignocellulose pretreatment using a newly isolated fungus, Amorphotheca resinae ZN1, and the consequent ethanol fermentation. Biotechnology for Biofuels., 3(47), 26.
Sluiter, A., Hames, B., Ruiz, R., Scarlata, C., Sluiter, J., & Templeton, D. (2008). Determination of sugars, byproducts, and degradation products in liquid fraction process samples. NREL/TP-510-42623. Golden: National Renewable Energy Laboratory.
Sluiter, A., Hames, B., Ruiz, R., Scarlata, C., Sluiter, J., Templeton, D., & Crocker, D. (2008). Determination of structural carbohydrates and lignin in biomass. NREL/TP-510-42618. Golden: National Renewable Energy Laboratory.
Adney, B., & Baker, J. (1996). Measurement of cellulase activities, laboratory analytical procedure (LAP). LAP-006. Golden: National Renewable Energy Laboratory.
Ghose, T. K. (1987). Measurement of cellulase activities. Pure and Applied Chemistry., 59(2), 257–268.
He, Y., Zhang, J., & Bao, J. (2014). Dry dilute acid pretreatment by co-currently feeding of corn Stover feedstock and dilute acid solution without impregnation. Bioresource Technology., 158(4), 360–364.
He, Y., Zhang, J., & Bao, J. (2016). Acceleration of biodetoxification on dilute acid pretreated lignocellulose feedstock by aeration and the consequent ethanol fermentation evaluation. Biotechnology for Biofuels., 9(1), 1–13.
Zhang, J., Chu, D., Huang, J., Yu, Z., Dai, G., & Bao, J. (2010). Simultaneous saccharification and ethanol fermentation at high corn stover solids loading in a helical stirring bioreactor. Biotechnology and Bioengineering., 105(4), 718–728.
China National Standards, (1989). Water quality-Determination of the chemical oxygen demand-potassium dichromate method of. Series number #11914–1989
Miller, G. L. (1959). Use of dinitrosalicylic acid reagent for determination of reducing sugar. Analytical Chemistry., 31(3), 426–428.
Ainsworth, E. A., & Gillespie, K. M. (2007). Estimation of total phenolic content and other oxidation substrates in plant tissues using Folin–Ciocalteu reagent. Nature Protocol., 2(4), 875–877.
Folch, J., Lee, M., & Sloane-Stanle, G. H. (1957). A simple method for the isolation and purification of total lipids from animal tissues. Journal of Biological Chemistry., 226(1), 497–509.
Zhang, T., Chi, Z. M., & Sheng, J. (2009). A highly thermosensitive and permeable mutant of the marine yeast Cryptococcus aureus G7a potentially useful for single-cell protein production and its nutritive components. Marine Biotechnology., 11(2), 280–286.
Xiong, L., Huang, C., Li, X., Chen, X., Wang, B., Wang, C., Zeng, X., & Chen, X. (2015). Acetone-butanol-ethanol (ABE) fermentation wastewater treatment by oleaginous yeast Trichosporon cutaneum. Applied Biochemistry and Biotechnolog., 176(2), 1–9.
Ramos, M. J., Fernández, C. M., Casas, A., Rodríguez, L., & Pérez, A. (2009). Influence of fatty acid composition of raw materials on biodiesel properties. Bioresource Technology., 100(1), 261–268.
Chen, X. F., Huang, C., Xiong, L., Chen, X., Chen, Y., & Ma, L. (2012). Oil production on wastewaters after butanol fermentation by oleaginous yeast Trichosporon coremiiforme. Bioresource Technology., 118(8), 594–597.
Author information
Authors and Affiliations
Corresponding authors
Electronic supplementary material
ESM 1
(DOCX 26 kb)
Rights and permissions
About this article
Cite this article
Wang, J., Hu, M., Zhang, H. et al. Converting Chemical Oxygen Demand (COD) of Cellulosic Ethanol Fermentation Wastewater into Microbial Lipid by Oleaginous Yeast Trichosporon cutaneum . Appl Biochem Biotechnol 182, 1121–1130 (2017). https://doi.org/10.1007/s12010-016-2386-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-016-2386-z