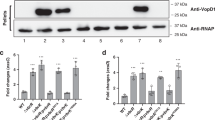
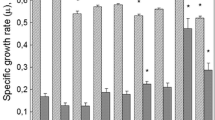
Abstract
Vibrio cholerae, the causative agent of cholera, poses serious threats to humans worldwide. V. cholerae faces host inflammatory response and encounters nitrosative stress before establishing successful colonization. It is not clear how V. cholerae combats nitric oxide and reactive nitrogen species. In the present study, we used three clinical strains of V. cholerae and tested their nitrosative stress response pattern towards sodium nitroprusside (SNP) and S-Nitrosoglutathione (GSNO). Among them, V. cholerae, belonging to both O1 and O139 serotypes, showed moderate resistance to SNP and GSNO. However, a V. cholerae strain belonging to non O1 and non O139 showed sensitivity to SNP but resistance towards GSNO. Reduced glutathione and glutathione reductase play a significant role to combat nitrosative stress in V. cholerae. This is the first report where we show the presence of GSNO reductase activity in V. cholerae and that it plays an important role to detoxify S-Nitrosoglutathione. GSNO reductase activity of V. cholerae was regulated by posttranslational modification through S-nitrosylation under in vitro conditions which could be reversed by dithiothreitol (DTT). In addition, we show that biofilm formation remained unaffected under nitrosative stress in V. cholerae.






Similar content being viewed by others
Reference
Ramamurthy, T., Bag, P. K., Pal, A., Bhattacharya, S. K., Bhattacharya, M. K., Shimada, T., Takeda, T., Karasawa, T., Kurazono, H., Takeda, Y., et al. (1993). Virulence patterns of Vibrio cholerae non-O1 strains isolated from hospitalised patients with acute diarrhoea in Calcutta, India. Journal of Medical Microbiology, 39, 310–317.
Kaper, J. B., Morris Jr., J. G., & Levine, M. M. (1995). Cholera. Clinical Microbiology Reviews, 8, 48–86.
Rothenbacher, F. P., & Zhu, J. (2014). Efficient responses to host and bacterial signals during Vibrio cholerae colonization. Gut Microbes, 5, 120–128.
Carpenter, A. W., & Schoenfisch, M. H. (2012). Nitric oxide release: part II. Therapeutic applications. Chemical Society Reviews, 41, 3742–3752.
Schairer, D. O., Chouake, J. S., Nosanchuk, J. D., & Friedman, A. J. (2012). The potential of nitric oxide releasing therapies as antimicrobial agents. Virulence, 3, 271–279.
D'Autreaux, B., Touati, D., Bersch, B., Latour, J. M., & Michaud-Soret, I. (2002). Direct inhibition by nitric oxide of the transcriptional ferric uptake regulation protein via nitrosylation of the iron. Proceedings of the National Academy of Sciences of the United States of America, 99, 16619–16624.
Keszler, A., Zhang, Y., & Hogg, N. (2010). Reaction between nitric oxide, glutathione, and oxygen in the presence and absence of protein: how are S-nitrosothiols formed? Free Radical Biology & Medicine, 48, 55–64.
Gardner, P. R., Costantino, G., Szabo, C., & Salzman, A. L. (1997). Nitric oxide sensitivity of the aconitases. The Journal of Biological Chemistry, 272, 25071–25076.
Kow, Y. W. (2002). Repair of deaminated bases in DNA. Free Radical Biology & Medicine, 33, 886–893.
Reiter, C. D., Teng, R. J., & Beckman, J. S. (2000). Superoxide reacts with nitric oxide to nitrate tyrosine at physiological pH via peroxynitrite. The Journal of Biological Chemistry, 275, 32460–32466.
Sahoo, R., Dutta, T., Das, A., Sinha Ray, S., Sengupta, R., & Ghosh, S. (2006). Effect of nitrosative stress on Schizosaccharomyces pombe: inactivation of glutathione reductase by peroxynitrite. Free Radical Biology & Medicine, 40, 625–631.
Janoff, E. N., Hayakawa, H., Taylor, D. N., Fasching, C. E., Kenner, J. R., Jaimes, E., & Raij, L. (1997). Nitric oxide production during Vibrio cholerae infection. The American Journal of Physiology, 273, G1160–G1167.
Qadri, F., Raqib, R., Ahmed, F., Rahman, T., Wenneras, C., Das, S. K., Alam, N. H., Mathan, M. M., & Svennerholm, A. M. (2002). Increased levels of inflammatory mediators in children and adults infected with Vibrio cholerae O1 and O139. Clinical and Diagnostic Laboratory Immunology, 9, 221–229.
Rabbani, G. H., Islam, S., Chowdhury, A. K., Mitra, A. K., Miller, M. J., & Fuchs, G. (2001). Increased nitrite and nitrate concentrations in sera and urine of patients with cholera or shigellosis. The American Journal of Gastroenterology, 96, 467–472.
Stern, A. M., Hay, A. J., Liu, Z., Desland, F. A., Zhang, J., Zhong, Z., & Zhu, J. (2012). The NorR regulon is critical for Vibrio cholerae resistance to nitric oxide and sustained colonization of the intestines. MBio, 3, e00013–e00012.
Hart, T. W. (1985). Some observations concerning the S-nitroso and S-phenylsulphonyl derivatives of L-cysteine and glutathione. Tetrahedron Letters, 26, 2013–2016.
Akerboom, T. P., & Sies, H. (1981). Assay of glutathione, glutathione disulfide, and glutathione mixed disulfides in biological samples. Methods in Enzymology, 77, 373–382.
Liu, L., Hausladen, A., Zeng, M., Que, L., Heitman, J., & Stamler, J. S. (2001). A metabolic enzyme for S-nitrosothiol conserved from bacteria to humans. Nature, 410, 490–494.
Carlberg, I., & Mannervik, B. (1975). Purification and characterization of the flavoenzyme glutathione reductase from rat liver. The Journal of Biological Chemistry, 250, 5475–5480.
Hess, D. T., Matsumoto, A., Kim, S. O., Marshall, H. E., & Stamler, J. S. (2005). Protein S-nitrosylation: purview and parameters. Nature Reviews. Molecular Cell Biology, 6, 150–166.
O’Toole, G. A. (2011) Microtiter dish biofilm formation assay. J Vis Exp.
Stevanin, T. M., Moir, J. W., & Read, R. C. (2005). Nitric oxide detoxification systems enhance survival of Neisseria meningitidis in human macrophages and in nasopharyngeal mucosa. Infection and Immunity, 73, 3322–3329.
Stevanin, T. M., Poole, R. K., Demoncheaux, E. A., & Read, R. C. (2002). Flavohemoglobin Hmp protects Salmonella enterica serovar Typhimurium from nitric oxide-related killing by human macrophages. Infection and Immunity, 70, 4399–4405.
Frey, A. D., Farres, J., Bollinger, C. J., & Kallio, P. T. (2002). Bacterial hemoglobins and flavohemoglobins for alleviation of nitrosative stress in Escherichia coli. Applied and Environmental Microbiology, 68, 4835–4840.
Dutta, D., Chowdhury, G., Pazhani, G. P., Guin, S., Dutta, S., Ghosh, S., Rajendran, K., Nandy, R. K., Mukhopadhyay, A. K., Bhattacharya, M. K., Mitra, U., Takeda, Y., Nair, G. B., & Ramamurthy, T. (2013). Vibrio cholerae non-O1, non-O139 serogroups and cholera-like diarrhea, Kolkata, India. Emerging Infectious Diseases, 19, 464–467.
Bag, P. K., Bhowmik, P., Hajra, T. K., Ramamurthy, T., Sarkar, P., Majumder, M., Chowdhury, G., & Das, S. C. (2008). Putative virulence traits and pathogenicity of Vibrio cholerae non-O1, non-O139 isolates from surface waters in Kolkata, India. Applied and Environmental Microbiology, 74, 5635–5644.
Guerra, D., Ballard, K., Truebridge, I., & Vierling, E. (2016). S-nitrosation of conserved cysteines modulates activity and stability of S-Nitrosoglutathione reductase (GSNOR). Biochemistry, 55, 2452–2464.
Mukhopadhyay, P., Zheng, M., Bedzyk, L. A., LaRossa, R. A., & Storz, G. (2004). Prominent roles of the NorR and Fur regulators in the Escherichia coli transcriptional response to reactive nitrogen species. Proceedings of the National Academy of Sciences of the United States of America, 101, 745–750.
Ding, H., & Demple, B. (2000). Direct nitric oxide signal transduction via nitrosylation of iron-sulfur centers in the SoxR transcription activator. Proceedings of the National Academy of Sciences of the United States of America, 97, 5146–5150.
Lee, J. H., Lee, K. L., Yeo, W. S., Park, S. J., & Roe, J. H. (2009). SoxRS-mediated lipopolysaccharide modification enhances resistance against multiple drugs in Escherichia coli. Journal of Bacteriology, 191, 4441–4450.
Lee, P. E., Demple, B., & Barton, J. K. (2009). DNA-mediated redox signaling for transcriptional activation of SoxR. Proceedings of the National Academy of Sciences of the United States of America, 106, 13164–13168.
Husain, M., Jones-Carson, J., Song, M., McCollister, B. D., Bourret, T. J., & Vazquez-Torres, A. (2010). Redox sensor SsrB Cys203 enhances Salmonella fitness against nitric oxide generated in the host immune response to oral infection. Proceedings of the National Academy of Sciences of the United States of America, 107, 14396–14401.
Hausladen, A., Privalle, C. T., Keng, T., DeAngelo, J., & Stamler, J. S. (1996). Nitrosative stress: activation of the transcription factor OxyR. Cell, 86, 719–729.
Camargo, A. C., de Paula, O. A., Todorov, S. D., & Nero, L. A. (2016). In vitro evaluation of Bacteriocins activity against Listeria monocytogenes biofilm formation. Applied Biochemistry and Biotechnology, 178, 1239–1251.
Ezzine, A., Moussaoui, M., El Hammi, E., Marzouki, M. N., & Baciou, L. (2014). Antimicrobial agents act differently on Staphyloccocus aureus and Ralstonia eutropha flavohemoglobins. Applied Biochemistry and Biotechnology, 173, 1023–1037.
Acknowledgements
We thank DBT, DBT-IPLS, UPE, and UGC CAS Phase II Government of India, CU-CRNN for providing infrastructural facility, DBT, Government of India for providing fellowship to Sourav Kumar Patra. We thank Mr. Chinmay Saha for his assistance in preparing the sequence alignment.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Patra, S.K., Bag, P.K. & Ghosh, S. Nitrosative Stress Response in Vibrio cholerae: Role of S-Nitrosoglutathione Reductase. Appl Biochem Biotechnol 182, 871–884 (2017). https://doi.org/10.1007/s12010-016-2367-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-016-2367-2