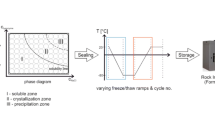
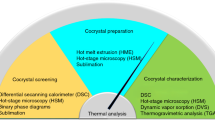
Abstract
The study of enzyme function often involves a multi-disciplinary approach. Several techniques are documented in the literature towards determining secondary and tertiary structures of enzymes, and X-ray crystallography is the most explored technique for obtaining three-dimensional structures of proteins. Knowledge of three-dimensional structures is essential to understand reaction mechanisms at the atomic level. Additionally, structures can be used to modulate or improve functional activity of enzymes by the production of small molecules that act as substrates/cofactors or by engineering selected mutants with enhanced biological activity. This paper presentes a short overview on how to streamline sample preparation for crystallographic studies of treated enzymes. We additionally revise recent developments on the effects of pressurized fluid treatment on activity and stability of commercial enzymes. Future directions and perspectives on the the role of crystallography as a tool to access the molecular mechanisms underlying enzymatic activity modulation upon treatment in pressurized fluids are also addressed.
Similar content being viewed by others
References
Borzani, W., Lima, U. A., & Aquarone, E. (1975). Engenharia bioquímica. São Paulo: Edgard Blucher.
Lima, U. A., Aquarone, E., Borzani, W., & Schmidell, W. (2001). Biotecnologia industrial: Processos fermentativos e enzimáticos. São Paulo: Edgard Blüncher.
Gießauf, A., Magor, W., Steinberger, D. J., & Marr, R. (1999). A study of hydrolases stability in supercritical carbon dioxide (SC-CO2). Enzyme and Microbial Technology, 24, 577–583.
Gießauf, A., & Gamse, T. (2000). A simple process for increasing the specific activity of porcine pancreatic lipase by supercritical carbon dioxide treatment. Journal of Molecular Catalysis B: Enzymatic, 9, 57–64.
Bauer, C., Gamse, T., & Marr, R. (2001). Quality improvement of crude porcine pancreatic lipase preparations by treatment with humid supercritical carbon dioxide. Biochemical Engineering Journal, 9, 119–123.
Findrik, Z., Vasic-Racki, D., Primozic, M., Habulin, M., & Knez, Z. (2005). Enzymatic activity of L-amino acid oxidase from snake venom Crotalus adamanteus in supercritical CO2. Biocatalysis and Biotransformation, 23, 315–321.
Oliveira, D., Feihrmann, A. C., Rubira, A. F., Kunita, M. H., Dariva, C., & Oliveira, J. V. (2006). Assessment of two immobilized lipases activity treated in compressed fluids. Journal of Supercritical Fluids, 38, 373–382.
Fricks, A. T., Souza, D. P. B., Oestreicher, E. G., Antunes, O. A. C., Girardi, J. S., Oliveira, D., & Dariva, C. (2006). Evaluation of radish (Raphanus sativus L.) peroxidase activity after high-pressure treatment with carbon dioxide. Journal of Supercritical Fluids, 38, 347–353.
Primo, M. S., Ceni, G. C., Marcon, N. S., Antunes, O. A. C., Oliveira, D., & Oliveira, J. V. (2007). Effects of compressed carbon dioxide treatment on the specificity of oxidase enzymatic complexes from mate tea leaves. Journal of Supercritical Fluids, 43, 283–290.
Andrade, J. M., Oestreicher, E. G., Oliveira, J. V., Oliveira, D., Antunes, O. A. C., & Dariva, C. (2008). Effect of treatment with compressed CO2 and propane on D-hydantoinase activity. Journal of Supercritical Fluids, 46, 342–350.
Eisenmenger, M. J., & Reyes-de-Corcuera, J. I. (2009). High hydrostatic pressure increased stability and activity of immobilized lipase in hexane. Enzyme and Microbial Technology, 45, 118–125.
Wang, S. S.-S., Chao, H.-S., Liu, H.-L., & Liu, H.-S. (2009). Stability of hen egg white lysozyme during denaturation is enhanced by pretreatment with supercritical carbon dioxide. Journal of Bioscience and Bioengineering, 107, 35–359.
Franken, L. P. G., Marcon, N. S., Treichel, H., Oliveira, D., Freire, D. M. G., Dariva, C., Destain, J., & Oliveira, J. V. (2010). Effect of treatment with compressed propane on lipases hydrolytic activity. Food and Bioprocess Technology, 3, 511–520.
Kuhn, G., Marangoni, M., Freire, D. M. G., Soares, V. F., Godoy, M. G., Castro, A., Di Luccio, M., Treichel, H., Mazutti, M. A., Oliveira, D., & Oliveira, J. V. (2010). Esterification activities of non-commercial lipases after pre-treatment in pressurized propane. Journal of Chemical Technology and Biotechnology, 85, 839–844.
Kuhn, G., Coghetto, C., Treichel, H., Oliveira, D., & Oliveira, J. V. (2011). Effect of compressed fluids treatment on the activity of inulinase from Kluyveromyces marxianus NRRL Y-7571 immobilized in montmorillonite. Process Biochemistry, 46, 2286–2290.
Kuhn, G., Dalla Rosa, C., Silva, M. F., Treichel, H., Oliveira, D., & Oliveira, J. V. (2013). Synthesis of fructooligosaccharides from Aspergillus niger commercial inulinase immobilized in montmorillonite pretreated in pressurized propane and LPG. Applied Biochemistry and Biotechnology, 169, 750–760.
Manera, A. P., Kuhn, G., Polloni, A., Marangoni, M., Zabot, G., Kalil, S. J., Oliveira, D., Treichel, H., Oliveira, J. V., Mazutti, M. A., & Maugeri, F. (2011). Effect of compressed fluids treatment on the activity, stability and enzymatic reaction performance of β-galactosidase. Food Chemistry, 125, 1235–1240.
Senyay-Oncel, D., & Yesil-Celiktas, O. (2011). Activity and stability enhancement of α-amylase treated with sub- and supercritical carbon dioxide. Journal of Bioscience and Bioengineering, 112, 435–440.
Braga, A. R. C., Silva, M. F., Oliveira, J. V., Treichel, H., & Kalil, S. J. (2012). Effect of compressed fluids treatment on β-galactosidase activity and stability. Bioprocess and Biosystems Engineering, 35, 1541–1547.
Liu, Y., Chen, D., Xu, L., & Yan, Y. (2012). Evaluation of structure and hydrolysis activity of Candida rugosa Lip7 in presence of sub-/super-critical CO2. Enzyme and Microbial Technology, 51, 354–358.
Liu, Y., Chen, D., & Wang, S. (2013). Effect of sub- and super-critical CO2 pretreatment on conformation and catalytic properties evaluation of two commercial enzymes of CALB and lipase PS. Journal of Chemical Technology and Biotechnology, 88, 1750–1756.
Liu, Y., Chen, D., & Yan, Y. (2013). Effect of ionic liquids, organic solvents and supercritical CO2 pretreatment on the conformation and catalytic properties of Candida rugosa lipase. Journal of Molecular Catalysis B: Enzymatic, 90, 123–127.
Silva, M. F., Golunski, S. M., Rigo, D., Mossi, V., Di Luccio, M., Mazutti, M. A., Oliveira, D., Oliveira, J. V., & Treichel, H. (2012). Pressurized propane: an alternative technique to increase inulinase activity. Industrial Biotechnology, 8, 293–299.
Silva, M. F., Golunski, S. M., Rigo, D., Mossi, V., Di Luccio, M., Mazutti, M. A., Pergher, S. B. C., Oliveira, D., Oliveira, J. V., & Treichel, H. (2013). Liquefied petroleum gas as solvent medium for the treatment of immobilized inulinases. Journal of Chemical Technology and Biotechnology, 88, 280–286.
Silva, M. F., Rigo, D., Mossi, V., Dallago, R. M., Di Luccio, M., Henrick, P., Kuhn, G. O., Dalla Rosa, C., Oliveira, D., Oliveira, J. V., & Treichel, H. (2013). Evaluation of enzymatic activity of commercial inulinase from Aspergillus niger immobilized in polyurethane foam. Food and Bioproducts Processing, 91, 54–59.
Silva, M. F., Rigo, D., Mossi, V., Golunski, S. M., Kuhn, G. O., Di Luccio, M., Dallago, R. M., Oliveira, D., Oliveira, J. V., & Treichel, H. (2013). Enzymatic synthesis of fructooligosaccharides by inulinases from Aspergillus niger and Kluyveromyces marxianus NRRL Y-7571 in aqueous–organic médium. Food Chemistry, 138, 148–153.
Chen, D., Peng, C., Zhang, H., & Yan, Y. (2013). Assessment of activities and conformation of lipases treated with sub- and supercritical carbon dioxide. Applied Biochemistry and Biotechnology, 169, 2189–2201.
Chen, D., Zhang, H., Xu, J., & Yan, Y. (2013). Effect of sub- and supercritical CO2 treatment on the properties of Pseudomonas cepacia lipase. Enzyme and Microbial Technology, 53, 110–117.
Melgosa, R., Sanz, M. T., Solaesa, A. G., Bucio, S. L., & Beltrán, S. (2015). Enzymatic activity and conformational and morphological studies of four commercial lipases treated with supercritical carbon dioxide. Journal of Supercritical Fluids, 97, 51–62.
Wang, J., Cao, Y., Sun, B., Wang, C., & Yo, Y. (2011). Effect of ultrasound on the activity of alliinase from fresh garlic. Ultrasonics Sonochemistry, 18, 534–540.
Wang, Z., Lin, X., Li, P., Zhang, J., Wang, S., & Ma, H. (2012). Effects of low intensity ultrasound on cellulase pretreatment. Bioresource Technology, 117, 222–227.
Ma, H., Huang, L., Jia, J., He, R., Luo, L., & Zhu, W. (2011). Effect of energy-gathered ultrasound on Alcalase. Ultrasonics Sonochemistry, 18, 419–424.
Ceni, G., Silva, P. C., Lerin, L., Oliveira, J. V., Toniazzo, G., Treichel, H., Oestreicher, E. G., & Oliveira, D. (2011). Ultrasound-assisted enzymatic transesterification of methyl benzoate and glycerol to 1-glyceryl benzoate in organic solvent. Enzyme and Microbial Technology, 48, 169–174.
Batistella, L., Ustra, M. K., Richetti, A., Pergher, S. B. C., Treichel, H., Oliveira, J. V., Lerin, L., & Oliveira, D. (2012). Assessment of two immobilized lipases activity and stability to low temperatures in organic solvents under ultrasound-assisted irradiation. Bioprocess and Biosystems Engineering, 35, 351–358.
Batistella, L., Lerin, L. A., Brugnerotto, P., Danielli, A. J., Trentin, C. M., Popiolsky, A., Treichel, H., Oliveira, J. V., & Oliveira, D. (2012). Ultrasound-assisted lipase-catalyzed transesterification of soybean oil in organic solvent system. Ultrasonics Sonochemistry, 19, 452–458.
Souza, M., Mezadri, E. T., Zimmerman, E., Leaes, E. X., Bassaco, M. M., Dal Prá, V., Foletto, E., Cancellier, A., Terra, L. M., Jahn, S. L., & Mazutti, M. A. (2013). Evaluation of activity of a commercial amylase under ultrasound-assisted irradiation. Ultrasonics Sonochemistry, 20, 89–94.
Leaes, E. X., Lima, D., Miklasevicius, L., Ramon, A. P., Dal Prá, V., Bassaco, M. M., Terra, L. M., & Mazutti, M. A. (2013). Effect of ultrasound-assisted irradiation on the activities of α-amylase and amyloglucosidase. Biocatalysis and Agricultural Biotechnology, 2, 21–25.
Szabó, O. E., & Csiszár, E. (2013). The effect of low-frequency ultrasound on the activity and efficiency of a commercial cellulase enzyme. Carbohydrate Polymers, 98, 1483–1489.
Bashari, M., Eibaid, A., Wang, J., Tian, Y., Xu, X., & Jin, Z. (2013). Influence of low ultrasound intensity on the degradation of dextran catalyzed by dextranase. Ultrasonics Sonochemistry, 20, 155–161.
Yu, Z. L., Zeng, W. C., & Lu, X. L. (2013). Influence of ultrasound to the activity of tyrosinase. Ultrasonics Sonochemistry, 20, 805–809.
Yu, Z. L., Zeng, W. C., Zhang, W. H., Liao, X. P., & Shi, B. (2014). Effect of ultrasound on the activity and conformation of a-amylase, papain and pepsin. Ultrasonics Sonochemistry, 21, 930–936.
Silva, J. R. F., Cantelli, K. C., Tres, M. V., Dalla Rosa, C., Meirelles, M. A. A., Soares, M. B. A., Oliveira, D., Oliveira, J. V., Treichel, H., & Mazutti, M. A. (2013). Treatment with compressed liquefied petroleum gas and ultrasound to improve celulase activity. Biocatalysis and Agricultural Biotechnology, 2, 102–107.
Ran, J., Jia, S., Liu, Y., & Wu, S. (2009). Characterization of cellulase under various intensities of static magnetic fields. Catalysis Communications, 11, 91–95.
Liu, Y., Jia, S., Ran, J., & Wu, S. (2010). Effects of static magnetic field on activity and stability of immobilized α-amylase in chitosan bead. Catalysis Communications, 11, 364–367.
Mizuki, T., Watanabe, N., Nagaoka, Y., Fukushima, T., Morimoto, H., Usami, R., & Maekawa, T. (2010). Activity of an enzyme immobilized on superparamagnetic particles in a rotational magnetic field. Biochemical and Biophysical Research Communications, 393, 779–782.
Vashisth, A., & Nagarajan, S. (2010). Effect on germination and early growth characteristics in sunflower (Helianthus annuus) seeds exposed to static magnetic field. Journal of Plant Physiology, 167, 149–156.
Cakmaka, T., Cakmakb, Z. E., Dumlupinarc, R., & Tekinayd, T. (2012). Analysis of apoplastic and symplastic antioxidant system in shallot leaves: impacts of weak static electric and magnetic field. Journal of Plant Physiology, 169, 1066–1073.
Radharishnan, R., & Kumari, B. D. R. (2012). Pulsed magnetic field: a contemporary approach offers to enhance plant growth and yield of soybean. Plant Physiology and Biochemistry, 51, 139–144.
Zheng, M., Zhang, S., Ma, G., & Wang, P. (2011). Effect of molecular mobility on coupled enzymatic reactions involving cofactor regeneration using nanoparticle-attached enzymes. Journal of Biotechnology, 154, 274–280.
Zheng, M., Su, Z., Ji, X., Ma, G., Wang, P., & Zhang, S. (2013). Magnetic field intensified bi-enzyme system with in situ cofactor regeneration supported by magnetic nanoparticles. Journal of Biotechnology, 168, 212–217.
Lehninger, A. L., Nelson, D. L., & Cox, M. M. (2002). Princípios de Bioquímica (3rd ed.). São Paulo: Sarvier.
Stryer, L. (1995). Biochemistry. New York: Freeman.
Monhemi, H., & Housaindokht, M. R. (2012). How enzymes can remain active and stable in a compressed gas? New insights into the conformational stability of Candida antarctica lipase B in near-critical propane. Journal of Supercritical Fluids, 72, 161–167.
Monhemi, H., Housaindokht, M. R., Bozorgmehr, M. R., & Googheri, M. S. S. (2012). Enzyme is stabilized by a protection layer of ionic liquids in supercritical CO2: insights from molecular dynamic simulation. Journal of Supercritical Fluids, 69, 1–7.
Housaindokht, M. R., Bozorgmehr, M. R., & Monhemi, H. (2012). Structural behavior of Candida antarctica lipase B in water and supercritical carbon dioxide: a molecular dynamic simulation study. Journal of Supercritical Fluids, 63, 180–186.
Housaindokht, M. R., & Monhemi, H. (2013). The open lid conformation of the lipase is explored in the compressed gas: new insights from molecular dynamic simulation. Journal of Molecular Catalysis B: Enzymatic, 87, 135–138.
Kao, F.-J., Ekhorutomwen, S. A., & Sawan, S. P. (1997). Residual stability of lipase from Candida rugosa in hexane, supercritical CO2, and supercritical SF6. Biotechnology Techniques, 11, 849–852.
Bauer, C., Steinberger, D.-J., Schlauer, G., Gamse, T., & Marr, R. (2000). Activation and denaturation of hydrolases in dry and humid supercritical carbon dioxide (SC-CO2). Journal of Supercritical Fluids, 19, 79–86.
Habulin, M., & Knez, Z. (2001). Activity and stability of lipases from different sources in supercritical carbon dioxide and near-critical propane. Journal of Chemical Technology and Biotechnology, 76, 1260–1266.
Zhou, L., Wu, J., Hu, X., Zhi, X., & Liao, X. (2009). Alterations in the activity and structure of pectin methylesterase treated by high pressure carbon dioxide. Journal of Agricultural and Food Chemistry, 57, 1890–1895.
Liao, X., Zhang, Y., Bei, J., Hu, X., & Wu, J. (2009). Alterations of molecular properties of lipoxygenase induced by dense phase carbon dioxide. Innovative Food Science and Emerging Technologies, 10, 47–53.
Fricks, A. T., Oestreicher, E. G., Cardoso Filho, L., Feihrmann, A. C., Cordeiro, Y., & Dariva, O. A. C. (2009). Antunes, effects of compressed fluids on the activity and structure of horseradish peroxidase. Journal of Supercritical Fluids, 50, 162–168.
Wang, S. S.-S., Lai, J.-T., Huang, M.-S., Tai, C. Y., & Liu, H.-S. (2010). Deactivation of isoamylase and β-amylase in the agitated reactor under supercritical carbon dioxide. Bioprocess and Biosystems Engineering, 33, 1007–1015.
Hu, W., Zhang, Y., Wang, Y., Zhou, L., Leng, X., Liao, X., & Hu, X. (2011). Aggregation and homogenization, surface charge and structural change, and inactivation of mushroom tyrosinase in an aqueous system by subcritical/supercritical carbon dioxide. Langmuir, 27, 909–916.
Lichty, J. J., Malecki, J. L., Agnew, H. D., Michelson-Horowitz, D. J., & Tan, S. (2005). Comparison of affinity tags for protein purification. Protein Expression and Purification, 41, 98–105.
Terpe, K. (2003). Overview of tag protein fusions: from molecular and biochemical fundamentals to commercial systems. Applied Microbiology Biotechnology, 60, 523–533.
Skerra, A., & Schmidt, T. G. M. (2000). Use of the Strep-tag and streptavidin for detection and purification of recombinant proteins. Methods in Enzymology, 326, 271–304.
Schmidt, T. G. M. (2007). A Skerra, the Strep-tag system for one-step purification and high-affinity detection or capturing of proteins. Nature Protocols, 2, 1528–1535.
IBA–Solutions for life science. (2014). Expression and purification of proteins using Strep-tag® or Twin-Strep-tag®—a comprehensive manual. Available from www.iba-lifesciences.com/technical-support.html.
Harper, S., & Speicher, D. W. (2011). Purification of proteins fused to glutathione S-tranferase. Methods in Molecular Biology, 681, 259–280.
Block, H., Maertens, B., Spriestersbach, A., Brinker, N., Kubicek, J., Fabis, R., Labahn, J., & Schäfer, F. (2009). Immobilized-metal affinity chromatography (IMAC): a review. Methods in Enzymology, 463, 439–473.
Arnau, J., Lauritzen, C., Petersen, G. E., & Pedersen, J. (2005). Current strategies for the use of affinity tags and tag removal for the purification of recombinant proteins. Protein Expression and Purification, 48, 1–13.
Bonnerjea, J., Oh, S., Hoare, M., & Dunnill, P. (1986). Protein purification: the right step at the right time. Biotechnology, 4, 954–958.
Liu, H. F., Ma, J., Winter, C., & Bayer, R. (2010). Recovery and purification process development for monoclonal antibody production. mAbs Landes Bioscience, 2, 480–499.
Astefanei, A., Kok, W. T., Bäuerlein, P., Núñes, O., Galceran, M. T., De Voogt, P., & Schoenmakers, P. J. (2015). Characterization of aggregates of surface modified fullerenes by asymmetrical flow field-flow fractionation with multi-angle light scattering detection. Journal of Chromatography A, 1408, 197–206.
Vezocnik, V., Rebolj, K., Sitar, S., Ota, K., Tusek-Znidaric, M., Strus, J., Sepcic, K., Pahovnik, D., Macek, P., & Zagar, E. (2015). Size fractionation and size characterization of nanoemulsions of lipid droplets and large unilamellar lipid vesicles by asymmetric-flow field-flow fractionation/multi-angle light scattering and dynamic light scattering. Journal of Chromatography A, 1418, 185–191.
Wittgren, B., & Wahlund, K.-G. (1997). Fast molecular mass and size characterization of polysaccharides using asymmetrical flow field-flow fractionation-multiangle light scattering. Journal of Chromatography A, 760, 205–218.
Boivin, S., Kozak, S., & Meijers, R. (2013). Optimization of protein purification and characterization using Thermofluor screens. Protein Expression and Purification, 91, 192–206.
Giacovazzo, C., Monaco, H. L., Viterbo, D., Scordari, F., Gilli, G., Zanotti, G., & Catti, M. (1992). Fundamentals of crystallography. New York: Oxford University Press.
Rupp, B. (2010). Biomolecular crystallography: principles, practice, and application to structural biology. New York: Garland Science.
W. F. Azevedo Jr (2004). Cristalização de macromoléculas biológicas, Department of Physics, Institute of Biosciences and Exact Sciences, University of State of São Paulo, São José do Rio Preto.
Matthews, B. W. (1968). Solvent content of protein crystals. Journal of Molecular Biology, 33, 491–497.
Ducruix, A., & Giegé, R. (1992). Crystallization of nucleic acids and proteins (a practical approach). Oxford: Oxford University Press.
Suryanarayana, C., & Norton, M. G. (1998). X-ray diffraction: a practical approach. New York: Plenum Press.
Stout, G. H., & Jensen, L. H. (1968). X-ray structure determination: a practical guide. New York: Macmillan Publishing Company.
Rhodes, G. (1993). Crystallography made crystal clear: a guide for users of macromolecular models. Londres: Academic Press.
European Synchrotron Radiation Facility (ESRF), What is a synchrotron? Avaliable from: http://www.esrf.eu/about/synchrotron-science/synchrotron.
Stoddard, B. L. (1996). Intermediate trapping and Laue X-ray diffraction: potential for enzyme mechanism, dynamics, and inhibitor screening. Pharmacology & Therapeutics, 70, 215–256.
Garman, E. F., & Schneider, T. R. (1997). Macromolecular cryocrystallography. Journal of Applied Crystallography, 30, 211–237.
Rondeau, J. M., & Schreuder, H. (2003). Protein crystallography and drug discovery. In The practice of medicinal chemistry (2nd ed.). Amsterdam: Elsevier.
Rondeau, J. M., & Schreuder, H. (2008). Protein crystallography and drug discovery. In The practice of medicinal chemistry (3rd ed.). Amsterdam: Elsevier.
Winn, M. D., Ballard, C. C., Cowtan, K. D., Dodson, E. J., Emsley, P., Evans, P. R., Keegan, R. M., Krissinel, E. B., Leslie, A. G. W., McCoy, A. J., McNicholas, S. J., Murshudov, G. N., Pannu, N. S., Potterton, E. A., Powell, H. R., Read, R. J., Vagin, A., & Wilson, K. S. (2011). Overview of the CCP4 suite and current developments. Acta Crystallographica Section D, D67, 235–242.
Kabsch, W. (2010). XDS. Acta Crystallographica Section D, D66, 125–132.
McCoy, A. J., Grosse-Kunstleve, R. W., Adams, P. D., Winn, M. D., Storoni, L. C., & Read, R. J. (2007). Phaser crystallographic software. Journal of Applied Crystallography, 40, 658–674.
Emsley, P., & Cowtan, K. D. (2004). Coot: model-building tools for molecular graphics. Acta Crystallographica Section D, D60, 2126–2132.
Adams, P. D., Afonine, P. V., Bunkóczi, G., Chen, V. B., Davis, I. W., Echols, N., Headd, J. J., Hung, L. W., Kapral, G. J., Grosse-Kunstleve, R. W., McCoy, A. J., Moriarty, N. W., Oeffner, R., Read, R. J., Richardson, D. C., Terwilliger, T. C., & Zwart, P. H. (2010). PHENIX: a comprehensive python-based system for macromolecular structure solution. Acta Crystallographica Section D, D66, 213–221.
W. F. Azevedo Jr (2004). Refinamento cristalográfico de biomoléculas, Department of Physics, Institute of Biosciences and Exact Sciences, University of State of São Paulo, São José do Rio Preto, São José do Rio Preto.
Morris, A. L., MacArthur, M. W., Hutchinson, E. G., & Thornton, J. M. (1992). Stereochemical quality of protein structure coordinates. Proteins: Structure, Function and Bioinformatics, 12, 345–364.
World Wide Protein Data Bank (PDB). Avaliable from: http://www.pdb.org/index.html.
Dutta, S., & Berman, H. M. (2005). Large macromolecular complexes in the Protein Data Bank: a status report. Structure, 13, 381–388.
Kendrew, J. C., Bodo, G., Dintzis, H. M., Parrish, R. G., Wyckoff, H., & Phillips, D. C. (1958). 3-dimensional model of the myoglobin molecule obtained by X-ray analysis. Nature, 181, 662–666.
Perutz, M. F., Rossmann, M. G., Cullis, A. F., Muirhead, H., Will, G., & North, A. C. T. (1960). Structure of haemoglobin-3-dimensional Fourier synthesis at 5.5 Å resolution, obtained by X-ray analysis. Nature, 185, 416–422.
Pandey, A. (2004). Concise encyclopedia of bioresource technology. New York: The Haworth Reference Press.
Houde, A., Kademi, A., & Leblanc, D. (2004). Lipases and their industrial applications: an overview. Applied Biochemistry and Biotechnology, 118, 155–170.
Knez, Z. (2009). Enzymatic reactions in dense gases. Journal of Supercritical Fluids, 47, 357–372.
Liu, Y., Chen, D., Yan, Y., Peng, C., & Xu, L. (2011). Biodiesel synthesis and conformation of lipase from Burkholderia cepacia in room temperature ionic liquids and organic solvents. Bioresource Technology, 102, 10414–10418.
Lakowicz, J. R. (2006). Principles of fluorescence spectroscopy (3rd ed.). LLC, New York: Springer Science + Business Media.
Ma, F., & Hanna, M. A. (1999). Biodiesel production: a review. Bioresource Technology, 70, 1–15.
Meher, L. C., Sagar, D. V., & Naik, S. N. (2006). Technical aspects of biodiesel production by transesterification—a review. Renewable and Sustainable Energy Reviews, 10, 248–268.
Singh, S. P., & Singh, D. (2010). Biodiesel production through the use of different sources and characterization of oils and their esters as the substitute of diesel: a review. Renewable and Sustainable Energy Reviews, 14, 200–216.
Leung, D. Y. C., Wu, X., & Leung, M. K. H. (2010). A review on biodiesel production using catalyzed transesterification. Applied Energy, 87, 1083–1095.
Gray, K. A., Zhao, L., & Emptage, M. (2006). Bioethanol. Current Opinion in Chemical Biology, 10, 141–146.
Alvira, P., Tomás-Pejó, E., Ballesteros, M., & Negro, M. J. (2010). Pretreatment technologies for an efficient bioethanol production process based on enzymatic hydrolysis: a review. Bioresource Technology, 101, 4851–4861.
Balat, M. (2011). Production of bioethanol from lignocellulosic materials via the biochemical pathway: a review. Energy Conversion and Management, 52, 858–875.
Acknowledgements
The authors thank CNPq and CAPES for the financial support and scholarships. KFS was supported by the Dahlem International Network PostDoc grant from Freie Universität Berlin.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Feiten, M.C., Di Luccio, M., Santos, K.F. et al. X-Ray Crystallography as a Tool to Determine Three-Dimensional Structures of Commercial Enzymes Subjected to Treatment in Pressurized Fluids. Appl Biochem Biotechnol 182, 429–451 (2017). https://doi.org/10.1007/s12010-016-2336-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-016-2336-9