Abstract
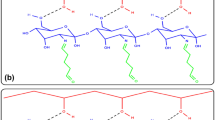
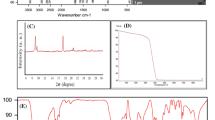
In this study, amine groups containing thiol-ene photocurable coating material for lipase immobilization were prepared. Lipase (EC 3.1.1.3) from Candida rugosa was immobilized onto the photocured coatings by physical adsorption and glutaraldehyde-activated covalent bonding methods, respectively. The catalytic efficiency of the immobilized and free enzymes was determined for the hydrolysis of p-nitrophenyl palmitate and also for the synthesis of p-nitrophenyl linoleate. The storage stability and the reusability of the immobilized enzyme and the effect of temperature and pH on the catalytic activities were also investigated. The optimum pH for free lipase and physically immobilized lipase was determined as 7.0, while it was found as 7.5 for the covalent immobilization. After immobilization, the optimum temperature increased from 37 °C (free lipase) to 50–55 °C. In the end of 15 repeated cycles, covalently bounded enzyme retained 60 and 70 % of its initial activities for hydrolytic and synthetic assays, respectively. While the physically bounded enzyme retained only 56 % of its hydrolytic activity and 67 % of its synthetic activity in the same cycle period. In the case of hydrolysis V max values slightly decreased after immobilization. For synthetic assay, the V max value for the covalently immobilized lipase was found as same as free lipase while it decreased dramatically for the physically immobilized lipase. Physically immobilized enzyme was found to be superior over covalent bonding in terms of enzyme loading capacity and optimum temperature and exhibited comparable re-use values and storage stability. Thus, a fast, easy, and less laborious method for lipase immobilization was developed.










Similar content being viewed by others
References
Villalonga, R., Fujii, A., Shinohara, H., Tachibana, S., & Asano, Y. (2008). Covalent immobilization of phenylalanine dehydrogenase on cellulose membrane for biosensor construction. Sensors and Actuators B, 129, 195–199.
Kadima, T., & Pickard, M. (1990). Immobilization of chloroperoxidase on aminopropyl-glass. Applied Environmental Microbiology, 56, 3473–3477.
Iyer, P. V., & Ananthanarayan, L. (2008). Enzyme stability and stabilization—aqueous and non-aqueous environment. Process Biochemistry, 43, 1019–1032.
Wang, Z.-G., Wan, L.-S., Liu, Z.-M., Huang, X.-J., & Xu, Z.-K. (2009). Enzyme immobilization on electrospun polymer nanofibers: an overview. Journal of Molecular Catalysis B: Enzymatic, 56, 189–195.
Tischer, W., & Wedekind, F. (1999). Immobilized enzymes: methods and applications. Topics in Current Chemistry, 200, 95–126.
Sheldon, R. A. (2007). Enzyme immobilization: the quest for optimum performance. Advanced Synthesis & Catalysis, 349, 1289–1307.
Bajpai, A. K., & Bhanu, S. (2003). Immobilization of alpha-amylase in vinyl-polymer-based interpenetrating polymer networks. Colloid and Polymer Science, 282, 76–83.
Guisan, J. M. (2006). Immobilization of enzymes and cells. Totowa, New Jersey: Humana Press Inc.
Mateo, C., Palomo, J. M., Fernandez-Lorente, G., Guisan, J. M., & Fernandez-Lafuente, R. (2007). Improvement of enzyme activity, stability and selectivity via immobilization techniques. Enzyme and Microbial Technology, 40, 1451–1463.
Pujari, N. S., Vaidya, B. K., Bagalkote, S., Ponrathnam, S., & Nene, S. (2006). Poly(urethane methacrylate-co-glycidyl methacrylate)-supported-polypropylene biphasic membrane for lipase immobilization. Journal of Membrane Science, 285, 395–403.
Hasirci, N., Aksoy, S., & Tumturk, H. (2006). Activation of poly(dimer acid-co-alkyl polyamine) particles for covalent immobilization of a-amylase. Reactive and Functional Polymers, 66, 1546–1551.
Goddard, J. M., & Hotchkiss, J. H. (2007). Polymer surface modification for the attachment of bioactive compounds. Progress in Polymer Science, 32, 698–725.
Turunc, O., Kahraman, M. V., Akdemir, Z. S., Kayaman-Apohan, N., & Gungor, A. (2009). Immobilization of a-amylase onto cyclic carbonate bearing hybrid material. Food Chemistry, 112, 992–997.
Dos Santos, J. C. S., Barbosa, O., Ortiz, C., Berenguer-Murcia, A., Rodrigues, R. C., & Fernandez-Lafuente, R. (2015). Importance of the support properties for immobilization or purification of enzymes. ChemCatChem, 7, 2413–2432.
Kazlauskas, R. J., & Bornscheuer, U. T. (1998). Biotechnology set. In H. J. Rehm, G. Pihler, A. Stadler, & P. J. W. Kelly (Eds.), (pp. 37–192). Weinheim, Germany: Wiley-VCH Verlag GmbH.
Hung, T.-C., Giridhar, R., Chiou, S.-H., & Wu, W.-T. (2003). Binary immobilization of Candida rugosa lipase on chitosan. Journal of Molecular Catalysis B: Enzymatic, 26, 69–78.
Sharma, R., Chisti, Y., & Banerjee, U. C. (2001). Production, purification, characterization, and applications of lipases. Biotechnology Advances, 19, 627–662.
Kent, J. A. (2007). Kent and Riegel’s handbook of industrial chemistry and biotechnology. Boston: Springer US.
Verger, R. (1997). Interfacial activation of lipases: facts and artifacts. Trends in Biotechnology, 15, 32–38.
Reis, P., Holmberg, K., Watzke, H., Leser, M. E., & Miller, R. (2009). Lipases at interfaces: a review. Advances in Colloid and Interface Science, 148, 237–250.
Derewenda, U., Brzozowski, A. M., Lawson, D. M., & Derewenda, Z. S. (1992). Catalysis at the interface: the anatomy of a conformational change in a triglyceride lipase. Biochemistry, 31, 1532–1541.
Derewenda, Z. S., Derewenda, U., & Dodson, G. G. (1992). The crystal and molecular structure of the Rhizomucor miehei triacylglyceride lipase at 1.9 A resolution. Journal of Molecular Biology, 227, 818–839.
Omay, D. (2014). Immobilization of lipase onto a photo-crosslinked polymer network: characterization and polymerization applications. Biocatalysis and Biotransformation, 32, 132–140.
Dursun, B. Y., Cigil, A. B., Dongez, D., Kahraman, M. V., Ogan, A., & Demir, S. (2016). Preparation and characterization of sol–gel hybrid coating films for covalent immobilization of lipase enzyme. Journal of Molecular Catalysis B: Enzymatic, 127, 18–25.
Hoyle, C. E., Lee, T. Y., & Roper, T. (2004). Thiol–enes: chemistry of the past with promise for the future. Journal of Polymer Science: Part A: Polymer Chemistry, 42, 5301–5338.
Machado, T. O., Sayer, C., & Arauj, P. H. H. (2016). Thiol-ene polymerisation: a promising technique to obtain novel biomaterials. European Polymer Journal. doi:10.1016/j.eurpolymj.2016.02.025.
Cakmakci, E., Danis, O., Demir, S., Mulazim, Y., & Kahraman, M. V. (2013). Alpha-amylase immobilization on epoxy containing thiol-ene photocurable materials. Journal of Microbiology and Biotechnology, 23, 205–210.
Akoh, C. C., Lee, G. G., & Shaw, J. F. (2004). Protein engineering and applications of Candida rugosa lipase isoforms. Lipids, 39, 513–526.
Benjamin, S., & Pandey, A. (1998). Candida rugosa lipases: molecular biology and versatility in biotechnology. Yeast, 14, 1069–1087.
Silva, C. J. S. M., Sousa, F., Gübitz, G., & Paulo, A. C. (2004). Chemical modifications on proteins using glutaraldehyde. Food Technology and Biotechnology, 42, 51–56.
Wine, Y., Cohen-Hadar, N., Freeman, A., & Frolow, F. (2007). Elucidation of the mechanism and end products of glutaraldehyde crosslinking reaction by X-ray structure analysis. Biotechnology and Bioengineering, 98, 711–718.
Barbosa, O., Ortiz, C., Berenguer-Murcia, A., Torres, R., Rodrigues, R. C., & Fernandez-Lafuente, R. (2014). Glutaraldehyde in bio-catalysts design: a useful crosslinker and a versatile tool in enzyme immobilization. RSC Advances, 4, 1583–1600.
Migneault, I., Dartiguenave, C., Bertrand, M. J., & Waldron, K. (2004). Glutaraldehyde: behavior in aqueous solution, reaction with proteins, and application to enzyme crosslinking. BioTechniques, 37, 790–802.
Cakmakci, E., Cigil, A. B., Danıs, O., Demir, S., & Kahraman, M. V. (2014). Immobilization of alpha-amylase on aminated polyimide membrane: preparation, characterization, and properties. Starch/Stärke, 66, 274–280.
Bradford, M. M. (1976). A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Analytical Biochemistry, 72, 248–254.
Arıca, M. Y., & Bayramoǧlu, G. (2004). Reversible immobilization of tyrosinase onto polyethyleneimine-grafted and Cu(II) chelated poly(HEMA-co-GMA) reactive membranes. Journal of Molecular Catalysis B: Enzymatic, 27, 255–265.
Kimmins, S. D., Wyman, P., & Cameron, N. R. (2014). Amine-functionalization of glycidyl methacrylate-containing emulsion-templated porous polymers and immobilization of proteinase K for biocatalysis. Polymer, 55, 416–425.
Ozyilmaz, G. (2009). The effect of spacer arm on hydrolytic and synthetic activity of Candida rugosa lipase immobilized on silica gel. Journal of Molecular Catalysis B: Enzymatic, 56, 231–236.
Suescun, A., Rueda, N., dos Santos, J. C. S., Castillo, J. J., Ortiz, C., Torres, R., Barbosa, O., & Fernandez-Lafuente, R. (2015). Immobilization of lipases on glyoxyl-octyl supports: improved stability and reactivation strategies. Process Biochemistry, 50, 1211–1217.
Barbosa, O., Torres, R., Ortiz, C., & Fernandez-Lafuente, R. (2012). Versatility of glutaraldehyde to immobilize lipases: effect of the immobilization protocol on the properties of lipase B from Candida antarctica. Process Biochemistry, 47, 1220–1227.
Bayramoglu, G., & Arica, M. Y. (2008). Adsorption of Cr(VI) onto PEI immobilized acrylate-based magnetic beads: isotherms, kinetics and thermodynamics study. Chemical Engineering Journal, 139, 20–28.
Hernandez, K., & Fernandez-Lafuente, R. (2011). Control of protein immobilization: coupling immobilization and site-directed mutagenesis to improve biocatalyst or biosensor performance. Enzyme and Microbial Technology, 48, 107–122.
Rodrigues, R. C., Berenguer-Murcia, Á., & Fernandez-Lafuente, R. (2011). Coupling chemical modification and immobilization to improve the catalytic performance of enzymes. Advanced Synthesis & Catalysis, 353, 2216–2238.
Barbosa, O., Ortiz, C., Berenguer-Murcia, Á., Torres, R., Rodrigues, R. C., & Fernandez-Lafuente, R. (2015). Strategies for the one-step immobilization-purification of enzymes as industrial biocatalysts. Biotechnology Advances, 33, 435–456.
Balcão, V. M., & Vila, M. M. (2014). Structural and functional stabilization of protein entities: state-of-the-art. Advanced Drug Delivery Reviews, 14, 213–220.
Barbosa, O., Torres, R., Ortiz, C., Berenguer-Murcia, A., Rodrigues, R., & Fernández-Lafuente, R. (2013). Heterofunctional supports in enzyme immobilization: from traditional immobilization protocols to opportunities in tuning enzyme properties. Biomacromolecules, 14, 2433–2462.
Manoel, E. A., dos Santos, J. C., Freire, D. M., Rueda, N., & Fernandez-Lafuente, R. (2015). Immobilization of lipases on hydrophobic supports involves the open form of the enzyme. Enzyme and Microbial Technology, 71, 53–57.
Mendes, A. A., Freitas, L., de Carvalho, A. K. F., de Oliveira, P. C., & de Castro, H. F. (2011). Immobilization of a commercial lipase from Penicillium camembertii (lipase G) by different strategies. Enzyme Research, 2011, 1–8.
Temoçin, Z. (2013). Covalent immobilization of Candida rugosa lipase on aldehyde functionalized hydrophobic support and the application for synthesis of oleic acid ester. Journal of Biomaterials Science, Polymer Edition, 24, 1618–1635.
Manzano, M. F. G., & Igarzabal, C. I. A. (2011). Immobilization of lipase from Candida rugosa on synthesized hydrogel for hydrolysis reaction. Journal of Molecular Catalysis B: Enzymatic, 72, 28–35.
Zhu, W., Zhang, Y., Hou, C., Pan, D., He, J., & Zhu, H. (2016). Covalent immobilization of lipases on monodisperse magnetic microspheres modified with PAMAM-dendrimer. Journal of Nanoparticle Research, 18, 1–13.
Wang, F., Nie, T. T., Shao, L. L., & Cui, Z. (2014). Comparison of physical and covalent immobilization of lipase from Candida antarctica on polyamine microspheres of alkylamine matrix. Biocatalysis and Biotransformation, 32, 314–326.
Pereira, M. G., et al. (2015). Stabilization of the lipase of Hypocrea pseudokoningii by multipoint covalent immobilization after chemical modification and application of the biocatalyst in oil hydrolysis. Journal of Molecular Catalysis B: Enzymatic, 121, 82–89.
Wang, X.-Y., Jiang, X.-P., Li, Y., Zeng, S., & Zhang, Y.-W. (2015). Preparation Fe3O4@chitosan magnetic particles for covalent immobilization of lipase from Thermomyces lanuginosus. International Journal of Biological Macromolecules, 75, 44–50.
Cao, L. (2006). Carrier-bound immobilized enzymes principles, application and design. Weinheim: Wiley-VCH.
Mislovicová, D., Masárová, J., Vikartovská, A., Gemeiner, P., & Michalková, E. (2004). Biospecific immobilization of mannan–penicillin G acylase neoglycoenzyme on Concanavalin A-bead cellulose. Journal of Biotechnology, 110, 11–19.
Wang, W., Zhou, W., Li, J., Hao, D., Su, Z., & Ma, G. (2015). Comparison of covalent and physical immobilization of lipase in gigaporous polymeric microspheres. Bioprocess and Biosystems Engineering, 38, 2107–2115.
Karra-Châabouni, M., Bouaziz, I., Boufi, S., do Rego, A. M. B., & Gargouri, Y. (2008). Physical immobilization of Rhizopus oryzae lipase onto cellulose substrate: activity and stability studies. Colloids and Surfaces B: Biointerfaces, 66, 168–177.
Ye, P., Wang, X., Han, Z., Yang, J., & Wan, R. (2012). Lipase immobilization on functional group controlled surfaces. Advanced Materials Research, 441, 452–456.
Handayani, N., Loos, K., Wahyuningrum, D., Buchari, & Zulfikar, M. A. (2012). Immobilization of Mucor miehei lipase onto macroporous aminated polyethersulfone membrane for enzymatic reactions. Membranes, 2, 198–213.
Verma, M. L., Naebe, M., Barrow, C. J., & Puri, M. (2013). Enzyme immobilisation on amino-functionalised multi-walled carbon nanotubes: structural and biocatalytic characterisation. PloS One, 8, e73642.
Yi, S.-S., Noh, J.-M., & Lee, Y.-S. (2009). Amino acid modified chitosan beads: improved polymer supports for immobilization of lipase from Candida rugosa. Journal of Molecular Catalysis B: Enzymatic, 57, 123–129.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Çakmakçi, E., Muhsir, P. & Demir, S. Physical and Covalent Immobilization of Lipase onto Amine Groups Bearing Thiol-Ene Photocured Coatings. Appl Biochem Biotechnol 181, 1030–1047 (2017). https://doi.org/10.1007/s12010-016-2266-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-016-2266-6