Abstract
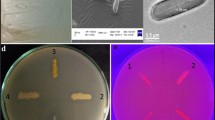
Low-temperature-tolerant microorganisms and their cold-active enzymes could be an innovative and invaluable tool in various industrial applications. In the present study, bacterial isolates from the sediment samples of Kongsfjord, Norwegian Arctic, were screened for β-galactosidase production. Among the isolates, KS25, KS85, KS60, and KS92 have shown good potential in β-galactosidase production at 20 °C. 16SrRNA gene sequence analysis revealed the relatedness of the isolates to Enterobacter ludwigii. The optimum growth temperature of the isolate was 25 °C. The isolate exhibited good growth and enzyme production at a temperature range of 15–35 °C, pH 5–10. The isolate preferred yeast extract and lactose for the maximum growth and enzyme production at conditions of pH 7.0, temperature of 25 °C, and agitation speed of 100 rpm. The growth and enzyme production was stimulated by Mn2+ and Mg2+ and strongly inhibited by Zn2+, Ni2+, and Cu+. β-Galactosidases with high specific activity at low temperatures are very beneficial in food industry to compensate the nutritional problem associated with lactose intolerance. The isolate exhibited a remarkable capability to utilize clarified whey, an industrial pollutant, for good biomass and enzyme yield and hence could be well employed in whey bioremediation.






Similar content being viewed by others
References
Cavicchioli, R., Charlton, T., Ertan, H., Omar, S. M., Siddiqui, K. S., & Williams, T. J. (2011). Biotechnological uses of enzymes from psychrophiles. Microbial Biotechnology, 4, 449–460.
Ghosh, M., Pulicherla, K. K., Rekha, V. P. B., Raja, P. K., & Sambasiva Rao, K. R. S. (2012). Cold active β-galactosidase from Thalassospira sp. 3SC-21 to use in milk lactose hydrolysis: a novel source from deep waters of Bay-of-Bengal. World Journal of Microbiology and Biotechnology, 28, 2859–2869.
Vester, J. K., Glaring, M. A., & Stougaard, P. (2014). Discovery of novel enzymes with industrial potential from a cold and alkaline environment by a combination of functional metagenomics and culturing. Microbial Cell Factories, 13, 72.
Nakagawa, T., Ikehata, R., Uchino, M., Miyaji, T., Takano, K., & Tomizuka, N. (2006). Cold-active acid β-galactosidase activity of isolated psychrophilic-basidiomycetous yeast Guehomyces pullulans. Microbiological Research, 161, 75–79.
Panesar, P. S., Kumari, S., & Panesar, R. (2010). Potential applications of immobilized β-galactosidase in food processing industries. Enzyme Research, 2010, 473137.
Białkowska, A. M., Cieśliński, H., Nowakowska, K. M., Kur, J., & Turkiewicz, M. (2009). A new β-galactosidase with a low temperature optimum isolated from the Antarctic Arthrobacter sp. 20B: gene cloning, purification and characterization. Archives of Microbiology, 191, 825–835.
Husain, Q. (2010). Beta galactosidases and their potential applications: a review. Critical Reviews in Biotechnology, 30(August 2009), 41–62.
Karasová-Lipovová, P., Strnad, H., Spiwok, V., Malá, Š., Králová, B., & Russell, N. J. (2003). The cloning, purification and characterisation of a cold-active β-galactosidase from the psychrotolerant Antarctic bacterium Arthrobacter sp. C2-2. Enzyme and Microbial Technology, 33, 836–844.
Sako, T., Matsumoto, K., & Tanaka, R. (1999). Recent progress on research and applications of non-digestible galactooligosaccharides. International Diary Journal, 9, 69–80.
Wierzbicka-Woś, A., Cieśliński, H., Wanarska, M., Kozłowska-Tylingo, K., Hildebrandt, P., & Kur, J. (2011). A novel cold-active β-D-galactosidase from the Paracoccus sp. 32d - gene cloning, purification and characterization. Microbial Cell Factories, 10, 108.
Horner, T. W., Dunn, M. L., Eggett, D. L., & Ogden, L. V. (2011). β-Galactosidase activity of commercial lactase samples in raw and pasteurized milk at refrigerated temperatures. Journal of Dairy Science, 94(2007), 3242–3249.
Nakagawa, T., Ikehata, R., Myoda, T., Miyaji, T., & Tomizuka, N. (2007). Overexpression and functional analysis of cold-active β-galactosidase from Arthrobacter psychrolactophilus strain F2. Protein Expression and Purification, 54, 295–299.
Coker, J., Sheridan, P. P., Loveland-Curtze, J., Gutshall, K. R., Auman, A. J., & Brenchley, J. E. (2003). Biochemical characterization of a β-galactosidase with a low temperature optimum obtained from an Antarctic Arthrobacter isolate. Journal of Bacteriology, 185(18), 5473–5482.
Hoyoux, A., Jennes, I., Dubois, P., Genicot, S., Dubail, F., François, J. M., Baise, E., Feller, G., & Gerday, C. (2001). Cold-adapted β-galactosidase from the antarctic psychrophile Pseudoalteromonas haloplanktis. Applied and Environmental Microbiology, 67(4), 1529–1535.
Fernandes, S., Geueke, B., Delgado, O., Coleman, J., & Hatti-Kaul, R. (2002). β-Galactosidase from a cold-adapted bacterium: purification, characterization and application for lactose hydrolysis. Applied Microbiology and Biotechnology, 58, 313–321.
Hu, J. M., Li, H., Cao, L. X., Wu, P. C., Zhang, C. T., Sang, S. L., Zhang, X. Y., Chen, M. J., Lu, J. Q., & Liu, Y. H. (2007). Molecular cloning and characterization of the gene encoding cold-active beta-galactosidase from a psychrotrophic and halotolerant Planococcus sp. L4. Journal of Agricultural and Food Chemistry, 55, 2217–2224.
Devi, R., Surendran, P. K., & Chakraborty, K. (2009). Antibiotic resistance and plasmid profiling of Vibrio parahaemolyticus isolated from shrimp farms along the Southwest coast of India. World Journal of Microbiology and Biotechnology, 25(1603), 2005–2012.
Silvester, R., Alexander, D., & Ammanamveetil, M. H. A. (2015). Prevalence, antibiotic resistance, virulence and plasmid profiles of Vibrio parahaemolyticus from a tropical estuary and adjoining traditional prawn farm along the southwest coast of India. Annals of Microbiology. doi:10.1007/s13213-015-1053-x.
Miller, J. H. (1972). Experiments in molecular genetics (pp. 352–355). Cold Spring Harbor: Cold Spring Harbor Laboratory.
Miller, J. H. (1992). A short course in bacterial genetics. Cold Spring Harbor: CSH Laboratory Press.
Ghatak, A., Guha, A. K., & Ray, L. (2010). β-D-Galactosidase from Enterobacter cloacae: production and some physicochemical properties. Applied Biochemistry and Biotechnology, 162, 1678–1688.
Zhang, L., Zhang, Y., Liu, Q., Meng, L., Hu, M., Lv, M., Li, K., Gao, C., Xu, P., & Ma, C. (2015). Production of diacetyl by metabolically engineered Enterobacter cloacae. Scientific Reports, 5, 9033.
Darah, I., Nisha, M., & Lim, S. H. (2013). Enhancement of polygalacturonase production from enterobacter aerogenes NBO2 by submerged fermentation. Advanced Studies in Biology, 5(5), 173–189.
Lu, L. L., Xiao, M., Li, Z. Y., Li, Y. M., & Wang, F. S. (2009). A novel transglycosylating β-galactosidase from Enterobacter cloacae B5. Process Biochemistry, 44, 232–236.
Lokapirnasari, W. P., Nazar, D. S., Nurhajati, T., Supranianondo, K., & Yulianto, A. B. (2015). Production and assay of cellulolytic enzyme activity of Enterobacter cloacae WPL 214 isolated from bovine rumen fluid waste of Surabaya abbatoir. Indonesia, 8, 367–371.
Mandal, S. M., Sharma, S., Pinnaka, A. K., Kumari, A., & Korpole, S. (2013). Isolation and characterization of diverse antimicrobial lipopeptides produced by Citrobacter and Enterobacter. BMC Microbiology, 13(1), 152.
Yamasaki, Y., Fukumoto, I., Kumagai, N., Ohta, Y., Nakagawa, T., Kawamukai, M., & Matsuda, H. (1992). Continuous chitosan hydrolyzate production by immobilized chitosanolytic enzyme from Enterobacter sp. G-1. Bioscience, Biotechnology, and Biochemistry, 56(June 2015), 1546–1551.
Banerjee, G., Pandey, S., Ray, A. K., & Kumar, R. (2015). Bioremediation of heavy metals by a novel bacterial strain Enterobacter cloacae and Its antioxidant enzyme activity, Flocculant production, and protein expression in presence of lead, cadmium, and nickel. Water, Air, & Soil Pollution, 226.
Shoebitz, M., Ribaudo, C. M., Pardo, M., Cantore, M. L., Ciampi, L., & Curá, J. (2009). Plant growth promoting properties of a strain of Enterobacter ludwigii isolated from Lolium perenne rhizosphere. Soil Biology and Biochemistry, 41, 1768–1774.
Kandasamy, P., Chaturvedi, N., Sisodia, B. S., Shasany, A. K., Gahoi, S., Marla, S. S., & Goel, R. (2013). Expression of CspE by a psychrotrophic bacterium Enterobacter ludwigii PAS1, isolated from Indian Himalayan soil and in silico protein modelling, prediction of conserved residues and active sites. Current Microbiology, 66, 507–514.
Ghashghaei, S., & Emtiazi, G. (2013). Production of calcite nanocrystal by a urease-positive strain of Enterobacter ludwigii and study of its structure by SEM. Current Microbiology, 67, 406–413.
Kumari, S., Panesar, P. S., Bera, M. B., & Singh, B. (2011). Permeabilization of Yeast Cells for beta galactosidase activity using mixture of organic solvants: a response surface methodology approach. Asian Journal of Biotechnology, 3(4), 406–414.
Choi, K. O., Song, S. H., & Yoo, Y. J. (2004). Permeabilization of Orchrobactrum anthropi SY509 cells with organic solvents for whole cell biocatalyst. Biotechnology and Bioprocess Engineering, 9, 147–150.
Sabu, A., Augur, C., Swati, C., & Pandey, A. (2006). Tannase production by Lactobacillus sp. ASR-S1 under solid-state fermentation. Process Biochemistry, 41, 575–580.
Baghel, V. S., Tripathi, R. D., Ramteke, P. W., Gopal, K., Dwivedi, S., Jain, R. K., Rai, U. N., & Singh, S. N. (2005). Psychrotrophic proteolytic bacteria from cold environment of Gangotri glacier, Western Himalaya, India. Enzyme and Microbial Technology, 36, 654–659.
Jadhav, V. V., Pote, S. S., Yadav, A., Shouche, Y. S., & Bhadekar, R. K. (2013). Extracellular cold active lipase from the psychrotrophic Halomonas sp. BRI 8 isolated from the Antarctic sea water. Songklanakarin Journal of Science and Technology, 35(6), 623–630.
Beniwal, V., Chhokar, V., Singh, N., & Sharma, J. (2010). Optimization of process parameters for the production of tannase and gallic acid by Enterobacter cloacae MTCC 9125. Journal of American Science, 6(July 2015), 389–397.
Alves, F. G., Filho, F. M., De Medeiros Burkert, J. F., & Kalil, S. J. (2010). Maximization of β-Galactosidase production: a simultaneous investigation of agitation and aeration effects. Applied Biochemistry and Biotechnology, 160, 1528–1539.
Zambare, V. P., Nilegaonkar, S. S., Kshirsagar, P. R., & Kanekar, P. P. (2014). Scale up production of Protease using Pseudomonas aeruginosa MCM B-327 and its detergent compatibility. Journal of Biochemical Technology, 5, 698–707.
Costa, E., Teixidó, N., Usall, J., Atarés, E., & Viñas, I. (2002). The effect of nitrogen and carbon sources on growth of the biocontrol agent Pantoea agglomerans strain CPA-2. Letters in Applied Microbiology, 35, 117–120.
Konsoula, Z., & Liakopoulou-Kyriakides, M. (2007). Co-production of α-amylase and β-galactosidase by Bacillus subtilis in complex organic substrates. Bioresource Technology, 98, 150–157.
Laxmi, N. P., Mutamed, M., & Nagendra, P. S. (2011). Effect of nitrogen sources on production of β-galactosidase from Bifidobacterium animalis Bb12 and Lactobacillus delbrueckii ssp. bulgaricus ATCC 11842 grown in whey under different culture conditions. International Food Research Journal, 18, 445–450.
Mahajan, P. M., Desai, K. M., & Lele, S. S. (2012). Production of cell membrane-bound α-and β-glucosidase by lactobacillus acidophilus. Food and Bioprocess Technology, 5, 706–718.
Khleifat, K. M., Abboud, M. M., Al-Mustafa, A. H., & Al-Sharafa, K. Y. (2006). Effects of carbon source and Vitreoscilla hemoglobin (VHb) on the production of β-galactosidase in Enterobacter aerogenes. Current Microbiology, 53, 277–281.
Soliman, N. A. (2008). Coproduction of thermostable amylase and β-galactosidase enzymes by Geobacillus stearothermophilus SAB-40: application of Plackett-Burman design to evaluate culture requirements affecting enzyme production. Journal of Microbiology and Biotechnology, 18, 695–703.
Nurullah, A. K. C. A. N. (2011). High level production of extracellular β-galactosidase from Bacillus licheniformis ATCC 12759 in submerged fermentation. African Journal of Microbiology Research, 5(26), 4615–4621.
Chan, V., Dreolini, L. F., Flintoff, K. A., Lloyd, S. J., & Mattenley, A. A. (2002). The effect of glycerol, glucose, glactose, lactose with galactose on the induction of -galactosidase in Escherichia coli. Journal of Experimental Microbiology and Immunology, 2(April), 130–137.
Panesar, P. S., Kennedy, J. F., Gandhi, D. N., & Bunko, K. (2007). Food chemistry Bioutilisation of whey for lactic acid production. Food Chemistry, 105, 1–14.
Foda, M. I., Dong, H., & Li, Y. (2010). Study the suitability of cheese whey for bio-butanol production by Clostridia. Journal of American Science, 6(July), 39–46.
Zohri, A. A., Gomah, N. H., & Ali, M. A. (2014). Utilization of cheese whey for bio-ethanol production. Universal Journal of Microbiology Research, 2(4), 57–69.
Acknowledgments
We are thankful to the Department of Marine Biology, Microbiology and Biochemistry and Sophisticated Testing and Instrumentation Centre at Cochin University of Science and Technology and National Centre for Antarctic and Ocean Research for providing the facilities and financial assistance to carry out the research.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Alikkunju, A.P., Sainjan, N., Silvester, R. et al. Screening and Characterization of Cold-Active β-Galactosidase Producing Psychrotrophic Enterobacter ludwigii from the Sediments of Arctic Fjord. Appl Biochem Biotechnol 180, 477–490 (2016). https://doi.org/10.1007/s12010-016-2111-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-016-2111-y