Abstract
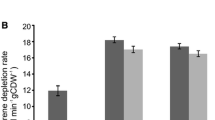
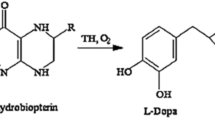
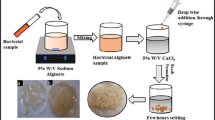
The present research work is concerned with the biotransformation of l-tyrosine to dopamine (DA) by calcium alginate entrapped conidiospores of a mutant strain of Aspergillus oryzae. Different strains of A. oryzae were isolated from soil. Out of 13 isolated strains, isolate-2 (I-2) was found to be a better DA producer. The wild-type I-2 was chemically improved by treating it with different concentrations of ethyl methyl sulfonate (EMS). Among seven mutant variants, EMS-6 exhibiting maximal DA activity of 43 μg/ml was selected. The strain was further exposed with l-cysteine HCl to make it resistant against diversion and environmental stress. The conidiospores of selected mutant variant A. oryzae EMS-6 strain were entrapped in calcium alginate beads. Different parameters for immobilization were investigated. The activity was further improved from 44 to 62 μg/ml under optimized conditions (1.5 % sodium alginate, 2 ml inoculum, and 2 mm bead size). The best resistant mutant variable exhibited over threefold increase in DA activity (62 μg/ml) than did wild-type I-2 (21 μg/ml) in the reaction mixture. From the results presented in the study, it was observed that high titers of DA activity in vitro could effectively be achieved by the EMS-induced mutagenesis of filamentous fungus culture used.





Similar content being viewed by others
References
Chakroborty, D., Sarkar, C., Yu, H., Wang, J., Liu, Z., Dasgupta, P. S., & Basu, S. (2011). Dopamine stabilizes tumor blood vessels by up-regulating angiopoietin 1 expression in pericytes and Kruppel-like factor-2 expression in tumor endothelial cells. Proceedings of the National Academy of Sciences, 108, 20730–20735.
Jackson, D. M., & Danielsson, A. W. (1994). Dopamine receptors: molecular biology, biochemistry and behavioural aspects. Pharmacology & Therapeutics, 64, 291–369.
Pahwa, R., & Koller, W. C. (1998). Advances in the treatment of Parkinson’s disease. Drugs Today (Barcelona, Spain), 34, 95–105.
Satoh, Y., Tajima, K., Munekata, M., Keasling, J. D., & Lee, T. S. (2012). Engineering of L tyrosine oxidation in Escherichia coli and microbial production of hydroxytyrosol. Metabolic Engineering, 14, 603–610.
Kurt, A. G., Aytan, E., Ozer, U., Ates, B., & Geckil, H. (2009). Production of L-dopa and dopamine in recombinant bacteria bearing the Vitreoscilla hemoglobin gene. Biotechnology Journal, 4, 1–12.
Tkacz, J. S., & Lange, L. (2004). Advances in fungal biotechnology for industry, agriculture and medicine (p. 242). New York: Kulwer Academic/Plenum.
Seetharam, G., & Saville, B. A. (2002). L-dopa production from tyrosinase immobilized on zeolite. Enzyme and Microbial Technology, 31, 747–753.
Peaston, R., & Weinkove, C. (2004). Measurement of catecholamines and their metabolites. Annals of Clinical Biochemistry, 41, 17–38.
Bradford, M. M. (1976). A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Analytical Biochemistry, 72, 248–254.
Arnow, L. E. (1937). Colorimetric determination of the components of 3, 4 dihydroxyphenylalanine tyrosine mixtures. Journal of Biological Chemistry, 118, 531–537.
Kandaswami, C., & Vaidyanathan, C. S. (1973). Oxidation of catechol in plants: purification and properties of the 3,4,3′,4′-tetrahydroxydiphenyl-forming enzyme system from Tacoma leaves. Journal of Biological Chemistry, 248, 4035–4039.
Snedecor, G. W., & Cochran, W. G. (1980). Statistical methods (7th ed.). Ames: Iowa State University Press.
Van, A. M. V. (1998). Mutation breeding: theory and practical application. Cambridge: Cambridge University Press. 152.
Karmakar, M., Ghosh, B., & Ray, R. R. (2012). Effect of extracellular factors on growth and dimorphism of Rhizopus oryzae with multiple enzyme synthesizing ability. Indian Journal of Microbiology, 2, 215–221.
Dave, R. I., & Shah, N. P. (1997). Effect of cysteine on the viability of yoghurt and probiotic bacteria in yoghurts made with commercial starter cultures. International Dairy Journal, 7, 537–545.
Zhou, L., Jiang, Y., Ma, L., He, Y., & Gao, J. (2015). Immobilization of glucose oxidase on polydopamine-functionalized graphene oxide. Applied Biochemistry and Biotechnology, 175, 1007–1017.
Mrudula, S., & Shyam, N. (2012). Immobilization of Bacillus megaterium MTCC 2444 by Ca-alginate entrapment method for enhanced alkaline protease production. Brazilian Archives of Biology and Technololgy, 55, 135–144.
Liu, X. D., Bao, D. C., Xue, W. M., Xiong, Y., Yu, W. T., Yu, X. J., Ma, X. J., & Yuan, Q. (2002). Preparation of uniform calcium alginate gel beads by membrane emulsification coupled with internal gelation. Journal of Applied Polymer Science, 87, 848–852.
Long, Y., Li, J., Zhao, T., Li, G., & Zhu, Y. (2015). A new arylalkylamine N-acetyltransferase in silkworm (Bombyx mori) affects integument pigmentation. Applied Biochemistry and Biotechnology, 175, 3447–3457.
Nedovic, V., & Willaert, R. (2005). Applications of cell immobilization biotechnology. New York: Springer.
Pialis, P., & Saville, B. A. (1998). Production of L-dopa from tyrosinase immobilized on Nylon 6, 6: enzyme stability and scale-up. Enzyme and Microbial Technology, 22, 261–268.
Surwase, S. N., Patil, S. A., Apine, O. A., & Jadhav, J. P. (2012). Efficient microbial conversion of L-tyrosine to L-dopa by Brevundimonas sp. SGJ. Applied Biochemistry and Biotechnology, 167, 1015–1028.
Majidi, D., & Aksoz, N. (2013). Stability of tyrosinase enzyme from Funalia trogii. American Journal of Microbiological Research, 1, 1–3.
Acknowledgments
The authors are grateful to Director IIB and Vice Chancellor of the University for providing research facilities and moral assistance.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ali, S., Nawaz, W. Biotransformation of l-tyrosine to Dopamine by a Calcium Alginate Immobilized Mutant Strain of Aspergillus oryzae . Appl Biochem Biotechnol 179, 1435–1444 (2016). https://doi.org/10.1007/s12010-016-2075-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-016-2075-y