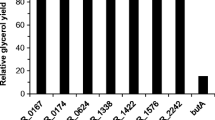
Abstract
To date, two types of glycerol dehydratases have been reported: coenzyme B12-dependent and coenzyme B12-independent glycerol dehydratases. The three-dimensional structure of the former is a dimer of αβγ heterotrimer, while that of the latter is a homodimer. Their mechanisms of reaction are typically enzymatic radical catalysis. Functional radical in both the glycerol dehydratases is the adenosyl radical. However, the adenosyl radical in the former originates from coenzyme B12 by homolytic cleavage, and that in the latter from S-adenosyl-methionine. Until some years ago, Clostridium butyricum VPI 1718 was the only microorganism known to possess B12-independent glycerol dehydratase, but since then, several other bacteria with this unique capability have been identified. This article focuses on the glycerol dehydratases and on 1,3-propanediol production from glycerol by naturally occurring and genetically engineered bacterial strains containing glycerol dehydratase.





Similar content being viewed by others
References
O’Brien, J. R., Raynaud, C., Croux, C., Girbal, L., Soucaille, P., & Lanzilotta, W. N. (2004). Insight into the mechanism of the B12-independent glycerol dehydratase from Clostridium butyricum: preliminary biochemical and structural characterization. Biochemistry, 43(16), 4635–4645.
Kraus, G. A. (2008). Synthetic methods for the preparation of 1,3-propanediol. CLEAN – Soil, Air, Water, 36(8), 648–651.
Hao, J., Lin, R., Zheng, Z., Liu, H., & Liu, D. (2008). Isolation and characterization of microorganisms able to produce 1,3-propanediol under aerobic conditions. World Journal of Microbiology and Biotechnology, 24(9), 1731–1740.
Jun, S. A., Moon, C., Kang, C. H., Kong, S. W., Sang, B. I., & Um, Y. (2010). Microbial fed-batch production of 1,3-propanediol using raw glycerol with suspended and immobilized Klebsiella pneumoniae. Applied Biochemistry and Biotechnology, 161(1–8), 491–501.
Huang, H., Gong, C. S., & Tsao, G. T. (2002). Production of 1,3-propanediol by Klebsiella pneumoniae. Applied Biochemistry and Biotechnology, 98–100, 687–698.
Clomburg, J. M., & Gonzalez, R. (2013). Anaerobic fermentation of glycerol: a platform for renewable fuels and chemicals. Trends in Biotechnology, 31(1), 20–28.
Dasari, M. A., Kiatsimkul, P.-P., Sutterlin, W. R., & Suppes, G. J. (2005). Low-pressure hydrogenolysis of glycerol to propylene glycol. Applied Catalysis A: general, 281(1), 225–231.
Durgapal, M., Kumar, V., Yang, T. H., Lee, H. J., Seung, D., & Park, S. (2014). Production of 1,3-propanediol from glycerol using the newly isolated Klebsiella pneumoniae J2B. Bioresource Technology, 159, 223–231.
Fan, X., Burton, R., & Zhou, Y. (2010). Glycerol (byproduct of biodiesel production) as a source for fuels and chemicals—mini review ~ !2009-08-26 ~ !2010-02-02 ~ !2010-04-09 ~ ! The Open Fuels & Energy Science Journal, 3(1), 17–22.
Pflugl, S., Marx, H., Mattanovich, D., & Sauer, M. (2014). Heading for an economic industrial upgrading of crude glycerol from biodiesel production to 1,3-propanediol by Lactobacillus diolivorans. Bioresource Technology, 152, 499–504.
Rossi, D. M., de Souza, E. A., Flores, S. H., & Ayub, M. A. Z. (2012). Bioconversion of residual glycerol from biodiesel synthesis into 1,3-propanediol and ethanol by isolated bacteria from environmental consortia. Renewable Energy, 39(1), 223–227.
de Souza, E. A., Rossi, D. M., & Ayub, M. A. Z. (2014). Bioconversion of residual glycerol from biodiesel synthesis into 1,3-propanediol using immobilized cells of Klebsiella pneumoniae BLh-1. Renewable Energy, 72, 253–257.
Renewables 2015 Global Status Report. REN21. c/o UNTP, 15 Rue de Milan, F-75441 Paris CEDEX 09, France ISBN 978-3-9815394-6-4.
ten Dam, J., & Hanefeld, U. (2011). Renewable chemicals: dehydroxylation of glycerol and polyols. ChemSusChem, 4(8), 1017–1034.
Ahrens, K., Menzel, K., Zeng, A. P., & Deckwer, W. D. (1998). Kinetic, dynamic, and pathway studies of glycerol metabolism by Klebsiella pneumoniae in anaerobic continuous culture: III. Enzymes and fluxes of glycerol dissimilation and 1,3-propanediol formation. Biotechnology and Bioengineering, 59(5), 544–552.
Toraya, T. (2000). Radical catalysis of B12 enzymes: structure, mechanism, inactivation, and reactivation of diol and glycerol dehydratases. Cellular and Molecular Life Sciences, 57(1), 106–127.
Toraya, T. (2003). Radical catalysis in coenzyme B12-dependent isomerization (eliminating) reactions. Chem Rev, 103(6), 2095–2127.
Raynaud, C., Sarcabal, P., Meynial-Salles, I., Croux, C., & Soucaille, P. (2003). Molecular characterization of the 1,3-propanediol (1,3-PD) operon of Clostridium butyricum. Proceedings of the National Academy of Sciences of the United States of America, 100(9), 5010–5015.
Liao, D. I., Dotson, G., Turner, I., Jr., Reiss, L., & Emptage, M. (2003). Crystal structure of substrate free form of glycerol dehydratase. Journal of Inorganic Biochemistry, 93(1–2), 84–91.
Yamanishi, M., Yunoki, M., Tobimatsu, T., Sato, H., Matsui, J., Dokiya, A., Iuchi, Y., Oe, K., Suto, K., Shibata, N., et al. (2002). The crystal structure of coenzyme B12-dependent glycerol dehydratase in complex with cobalamin and propane-1,2-diol. European Journal of Biochemistry, 269(18), 4484–4494.
Liu, Y., Gallo, A. A., Bajpai, R. K., Chistoserdov, A., Nelson, A. T., Segura, L. N., & Xu, W. (2010). The diversity and molecular modelling analysis of B12-dependent and B12-independent glycerol dehydratases. Int ernational Journal of Bioinformatics Research and Applications, 6(5), 484–507.
Shibata, N., Masuda, J., Tobimatsu, T., Toraya, T., Suto, K., Morimoto, Y., & Yasuoka, N. (1999). A new mode of B12 binding and the direct participation of a potassium ion in enzyme catalysis: X-ray structure of diol dehydratase. Structure, 7(8), 997–1008.
Sun, J., van den Heuvel, J., Soucaille, P., Qu, Y., & Zeng, A. P. (2003). Comparative genomic analysis of dha regulon and related genes for anaerobic glycerol metabolism in bacteria. Biotechnology Progress, 19(2), 263–272.
Seo, M. Y., Seo, J. W., Heo, S. Y., Baek, J. O., Rairakhwada, D., Oh, B. R., Seo, P. S., Choi, M. H., & Kim, C. H. (2009). Elimination of by-product formation during production of 1,3-propanediol in Klebsiella pneumoniae by inactivation of glycerol oxidative pathway. Applied Microbiology and Biotechnology, 84(3), 527–534.
Seifert, C., Bowien, S., Gottschalk, G., & Daniel, R. (2001). Identification and expression of the genes and purification and characterization of the gene products involved in reactivation of coenzyme B12-dependent glycerol dehydratase of Citrobacter freundii. European Journal of Biochemistry, 268(8), 2369–2378.
Padmakumar, R., Taoka, S., Padmakumar, R., & Banerjee, R. (1995). Coenzyme B12 is coordinated by histidine and not dimethylbenzimidazole on methylmalonyl-CoA mutase. Journal of the American Chemical Society, 117(26), 7033–7034.
Gonzalez-Pajuelo, M., Meynial-Salles, I., Mendes, F., Andrade, J. C., Vasconcelos, I., & Soucaille, P. (2005). Metabolic engineering of Clostridium acetobutylicum for the industrial production of 1,3-propanediol from glycerol. Metabolic Engineering, 7(5–6), 329–336.
Scott, K. P., Martin, J. C., Campbell, G., Mayer, C. D., & Flint, H. J. (2006). Whole-genome transcription profiling reveals genes up-regulated by growth on fucose in the human gut bacterium “Roseburia inulinivorans”. Journal of Bacteriology, 188(12), 4340–4349.
Tang, X., Tan, Y., Zhu, H., Zhao, K., & Shen, W. (2009). Microbial conversion of glycerol to 1,3-propanediol by an engineered strain of Escherichia coli. Applied and Environmental Microbiology, 75(6), 1628–1634.
Jiang, W., Wang, S., Yang, Z., & Fang, B. (2015). B12-independent glycerol dehydratase and its reactivase from Clostridia butyricum: Optimizing cloning by uniform design logic. Engineering in Life Sciences, 15(5), 519–524.
Frey, P. A. (2001). Radical mechanisms of enzymatic catalysis. Annual Review of Biochemistry, 70, 121–148.
Abend, A., Bandarian, V., Reed, G. H., & Frey, P. A. (2000). Identification of cis-ethanesemidione as the organic radical derived from glycolaldehyde in the suicide inactivation of dioldehydrase and of ethanolamine ammonia-lyase. Biochemistry, 39(20), 6250–6257.
Kozbial, P. Z., & Mushegian, A. R. (2005). Natural history of S-adenosylmethionine-binding proteins. BMC Structural Biology, 5, 19.
Frey, P. A., & Reed, G. H. (2000). Radical mechanisms in adenosylmethionine- and adenosylcobalamin-dependent enzymatic reactions. Archives of Biochemistry and Biophysics, 382(1), 6–14.
Layer, G., Kervio, E., Morlock, G., Heinz, D. W., Jahn, D., Retey, J., & Schubert, W. D. (2005). Structural and functional comparison of HemN to other radical SAM enzymes. Biological Chemistry, 386(10), 971–980.
Sofia, H. J., Chen, G., Hetzler, B. G., Reyes-Spindola, J. F., & Miller, N. E. (2001). Radical SAM, a novel protein superfamily linking unresolved steps in familiar biosynthetic pathways with radical mechanisms: functional characterization using new analysis and information visualization methods. Nucleic Acids Research, 29(5), 1097–1106.
Frey, P. A., Hegeman, A. D., & Ruzicka, F. J. (2008). The radical SAM superfamily. Critical Reviews in Biochemistry and Molecular Biology, 43(1), 63–88.
Hong, W. K., Kim, C. H., Heo, S. Y., Luo, L. H., Oh, B. R., Rairakhwada, D., & Seo, J. W. (2011). 1,3-Propandiol production by engineered Hansenula polymorpha expressing dha genes from Klebsiella pneumoniae. Bioprocess and Biosystems Engineering, 34(2), 231–236.
Selembo, P. A., Perez, J. M., Lloyd, W. A., & Logan, B. E. (2009). Enhanced hydrogen and 1,3-propanediol production from glycerol by fermentation using mixed cultures. Biotechnology and Bioengineering, 104(6), 1098–1106.
Anand, P., & Saxena, R. K. (2012). A comparative study of solvent-assisted pretreatment of biodiesel derived crude glycerol on growth and 1,3-propanediol production from Citrobacter freundii. New Biotechnology, 29(2), 199–205.
Liao, D. I., Reiss, L., Turner, I., & Dotson, G. (2003). Structure of glycerol dehydratase reactivase: a new type of molecular chaperone. Structure, 11(1), 109–119.
Jiang, W., Li, W., Hong, Y., Wang, S., & Fang, B. (2016). Cloning, expression, mutagenesis library construction of glycerol dehydratase, and binding mode simulation of its reactivase with ligands. Applied Biochemistry and Biotechnology, 178(4), 739–752.
Feliks, M., & Ullmann, G. M. (2012). Glycerol dehydratation by the B12-independent enzyme may not involve the migration of a hydroxyl group: a computational study. The Journal of Physical Chemistry B, 116(24), 7076–7087.
Kumar, S. R., & Chandrasekaran, M. (2003). Continuous production of l-glutaminase by an immobilized marine Pseudomonas sp BTMS-51 in a packed bed reactor. Process Biochemistry, 38(10), 1431–1436.
Pai, S.-L., Hsu, Y.-L., Chong, N.-M., Sheu, C.-S., & Chen, C.-H. (1995). Continuous degradation of phenol by Rhodococcus sp. immobilized on granular activated carbon and in calcium alginate. Bioresource Technology, 51(1), 37–42.
Siman, R., Borges, A., Ratusznei, S., Rodrigues, J., Zaiat, M., Foresti, E., & Borzani, W. (2004). Influence of organic loading on an anaerobic sequencing biofilm batch reactor (ASBBR) as a function of cycle period and wastewater concentration. Journal of Environmental Management, 72, 241–247.
Demick, J. M., & Lanzilotta, W. N. (2011). Radical SAM activation of the B12-independent glycerol dehydratase results in formation of 5′-deoxy-5′-(methylthio)adenosine and not 5′-deoxyadenosine. Biochemistry, 50(4), 440–442.
Lanz, N. D., & Booker, S. J. (2015). Auxiliary iron-sulfur cofactors in radical SAM enzymes. Biochimica et Biophysica Acta, 1853(6), 1316–1334.
Wagner, A. F., Frey, M., Neugebauer, F. A., Schäfer, W., & Knappe, J. (1992). The free radical in pyruvate formate-lyase is located on glycine-734. Proceedings of the National Academy of Sciences of the United States of America, 89(3), 996–1000.
Wagner, A. F., Schultz, S., Bomke, J., Pils, T., Lehmann, W. D., & Knappe, J. (2001). YfiD of Escherichia coli and Y06I of bacteriophage T4 as autonomous glycyl radical cofactors reconstituting the catalytic center of oxygen-fragmented pyruvate formate-lyase. Biochemical and Biophysical Research Communications, 285(2), 456–462.
Yang, G., Tian, J., & Li, J. (2007). Fermentation of 1,3-propanediol by a lactate deficient mutant of Klebsiella oxytoca under microaerobic conditions. Applied Microbiology and Biotechnology, 73(5), 1017–1024.
Szymanowska-Powalowska D, Leja K: An increasing of the efficiency of microbiological synthesis of 1,3-propanediol from crude glycerol by the concentration of biomass. In., vol. 17: scielocl; 2014: 72–78.
Saxena, R. K., Anand, P., Saran, S., & Isar, J. (2009). Microbial production of 1,3-propanediol: recent developments and emerging opportunities. Biotechnology Advances, 27(6), 895–913.
Ringel, A., Wilkens, E., Hortig, D., Willke, T., & Vorlop, K.-D. (2012). An improved screening method for microorganisms able to convert crude glycerol to 1,3-propanediol and to tolerate high product concentrations. Applied Microbiology and Biotechnology, 93(3), 1049–1056.
Gungormusler, M., Gonen, C., & Azbar, N. (2011). Continuous production of 1,3-propanediol using raw glycerol with immobilized Clostridium beijerinckii NRRL B-593 in comparison to suspended culture. Bioprocess and Biosystems Engineering, 34(6), 727–733.
da Silva, G. P., de Lima, C. J. B., & Contiero, J. (2015). Production and productivity of 1,3-propanediol from glycerol by Klebsiella pneumoniae GLC29. Catalysis Today, 257(Part 2), 259–266.
Drozdzynska, A., Pawlicka, J., Kubiak, P., Kosmider, A., Pranke, D., Olejnik-Schmidt, A., & Czaczyk, K. (2014). Conversion of glycerol to 1,3-propanediol by Citrobacter freundii and Hafnia alvei—newly isolated strains from the Enterobacteriaceae. New Biotechnology, 31(5), 402–410.
Jin, P., Li, S., Lu, S.-G., Zhu, J.-G., & Huang, H. (2011). Improved 1,3-propanediol production with hemicellulosic hydrolysates (corn straw) as cosubstrate: impact of degradation products on Klebsiella pneumoniae growth and 1,3-propanediol fermentation. Bioresource Technology, 102(2), 1815–1821.
Jin, P., Lu, S.-G., Huang, H., Luo, F., & Li, S. (2011). Enhanced reducing equivalent generation for 1,3-propanediol production through cofermentation of glycerol and xylose by Klebsiella pneumoniae. Applied Biochemistry and Biotechnology, 165(7–8), 1532–1542.
Cl, R., Lee, J., Sarcabal, P., Croux, C., Meynial-Salles, I., & Soucaille, P. (2011). Molecular characterization of the glycerol-oxidative pathway of Clostridium butyricum VPI 1718. Journal of Bacteriology, 193(12), 3127–3134.
Chatzifragkou, A., Dietz, D., Komaitis, M., Zeng, A.-P., & Papanikolaou, S. (2010). Effect of biodiesel-derived waste glycerol impurities on biomass and 1,3-propanediol production of Clostridium butyricum VPI 1718. Biotechnology and Bioengineering, 107(1), 76–84.
Kumar, V., Sankaranarayanan, M., Durgapal, M., Zhou, S., Ko, Y., Ashok, S., Sarkar, R., & Park, S. (2013). Simultaneous production of 3-hydroxypropionic acid and 1,3-propanediol from glycerol using resting cells of the lactate dehydrogenase-deficient recombinant Klebsiella pneumoniae overexpressing an aldehyde dehydrogenase. Bioresource Technology, 135, 555–563.
Zhao, Y.-N., Chen, G., & Yao, S.-J. (2006). Microbial production of 1,3-propanediol from glycerol by encapsulated Klebsiella pneumoniae. Biochemical Engineering Journal, 32(2), 93–99.
Oh, B., Hong, W., Heo, S., Luo, L., Seo, J., & Kim, C. (2013). The production of 1,3-propanediol from mixtures of glycerol and glucose by a Klebsiella pneumonia mutant deficient in carbon catabolite repression. Bioresource Technology, 130, 719–724.
Tobajas, M., Mohedano, A. F., Casas, J. A., & Rodríguez, J. J. (2009). Unstructured kinetic model for reuterin and 1,3-propanediol production by Lactobacillus reuteri from glycerol/glucose cofermentation. Journal of Chemical Technology & Biotechnology, 84(5), 675–680.
Chen, X., Zhang, D., Qi, W., Gao, S., Xiu, Z., & Xu, P. (2003). Microbial fed-batch production of 1, 3-propanediol by Klebsiella pneumonia under micro-aerobic conditions. Applied Microbiology and Biotechnology, 63, 143–146.
Cheng, K.-K., Liu, D.-H., Sun, Y., & Liu, W.-B. (2004). 1,3-Propanediol production by Klebsiella pneumonia Under different aeration strategies. Biotechnology Letters, 26, 911–915.
Jolly, J., Kitzmann, B., Ramalingam, S., & Ramachandran, K. (2014). Biosynthesis of 1,3-propanediol from glycerol with Lactobacillus reuteri: effect of operating variables. Journal of Bioscience and Bioengineering, 118(2), 188–194.
Rossi, D., Souza, E., Flôres, S., & Ayub, M. (2013). Conversion of residual glycerol from biodiesel synthesis into 1, 3-propanediol by a new strain of Klebsiella pneumoniae. Renewable Energy, 55, 404–409.
Casali, S., Gungormusler, M., Bertin, L., Fava, F., & Azbar, N. (2012). Development of a biofilm technology for the production of 1,3-propanediol (1,3-PDO) from crude glycerol. Biochemical Engineering Journal, 64, 84–90.
Hiremath, A., Kannabiran, M., & Rangaswamy, V. (2011). 1,3-Propanediol production from crude glycerol from Jatropha biodiesel process. New Biotechnoloty, 28, 19–23.
Metsoviti, M., Zeng, A., Koutinas, A., & Papanikolaou, S. (2013). Enhanced 1,3-propanediol production by a newly isolated Citrobacter freundii strain cultivated on biodiesel-derived waste glycerol through sterile and non-sterile bioprocesses. Journal of Bacteriology, 163(4), 408–418.
Jia, J., Yang, Y., & Yu, H. (2012). Phenol degradation characteristics and immobilization of Acinebobacte strain HY1. Advanced Materials Research, 549, 172–176.
Gungormusler-Yilmaz, M., Cicek, N., Levin, D. B., & Azbar, N. (2015). Cell immobilization for microbial production of 1,3-propanediol. Critical Review Biotechnology, 15, 1–13.
Kalme, S., Jadhav, S., Parshetti, G., & Govindwar, S. (2010). Biodegradation of Green HE4B: co-substrate effect, biotransformation enzymes and metabolite toxicity analysis. Indian Journal of Microbiology, 50, 156–164.
Oranusi, N., & Ogugbue, C. (2005). The effect of co-substrate on primary biodegradation of triphenylmethane dye by Pseudomonas sp. African Journal of Applied Zoology and Environmental Biology, 7, 38–44.
Park, J., Krumins, V., Kjellerup, B., Fennell, D., Rodenburg, L., Sower, K., Kerkhof, L., & Häggblom, M. (2011). The effect of co-substrate activation on indigenous and bioaugmented PCB dechlorinating bacterial communities in sediment microcosms. Applied Microbiology and Biotechnology, 89, 2005–2017.
Jeffries, T. W. (1983). Utilization of xylose by bacteria, yeasts, and fungi. Advances in Biochemical Engineering/Biotechnology, 27, 1–32.
Oh, B.-R., Seo, J.-W., Heo, S.-Y., Hong, W.-K., Luo, L., Kim, S., Park, D.-H., & Kim, C. (2012). Optimization of culture conditions for 1,3-propanediol production from glycerol using a mutant strain of Klebsiella pneumoniae. Applied Biochemistry and Biotechnology, 166(1), 127–137.
Sen, B., Dabir, A. P., Lanjekar, V. B., & Ranade, D. R. (2015). Isolation and partial characterization of a new strain of Klebsiella pneumonia capable of high 1,3-propanediol production from glycerol. Global Journal of Environment Science Management, 1(2), 99–108.
Wilkens, E., Ringel, A., Hortig, D., Willke, T., & Vorlop, W. (2012). High-level production of 1,3-propanediol from crude glycerol by Clostridium butyricum AKR102a. Applied Microbiology and Biotechnology, 93, 1057–1063.
Yen, H., Li, F., & Chang, J. (2014). 1,3-Propanediol and 2,3-butanediol produced from glycerol by an isolated indigenous Klebsiella sp. Ana-WS5. Bioresource Technology, 153, 374–378.
Liu, H., Zhang, D., Xu, Y. H., Mu, Y., Sun, Y., & Xiu, J. (2007). Microbial production of 1,3-propanediol from glycerol by Klebsiella pneumoniae under micro-aerobic conditions up to a pilot plant. Biotechnological Letters, 29, 1281–1285.
Anand, P., Saxena, R., Yadav, S., & Jahan, F. (2010). A greener solution for darker side of biodiesel: utilization of crude glycerol in 1,3-propanediol production. Journal of Biofuels, 1, 83–91.
Mu, Y., Teng, H., Zhang, D., Wang, W., & Xiu, Z. (2006). Microbial production of 1,3-propanediol by Klebsiella pneumonia using crude glycerol from biodiesel preparations. Biotechnological Letters, 28, 1755–1759.
Moon, C., Ahn, J.-H., Kim, S., Sang, B.-I., & Um, Y. (2010). Effect of biodiesel-derived raw glycerol on 1,3-propanediol production by different microorganisms. Applied Biochemistry and Biotechnology, 161(1–8), 502–510.
Chi, Z., Pyle, D., Wen, Z., Frear, C., & Chen, S. (2007). A laboratory study of producing docosahexaenoic acid from biodiesel-waste glycerol by microangal fermentation. Process Biochemistry, 42, 1537–1545.
Szymanowska-Powałowska, D. (2015). The effect of high concentrations of glycerol on the growth, metabolism and adaptation capacity of Clostridium butyricum DSP1. Electronic Journal of Biotechnology, 18(2), 128–133.
Menzel, K., Zeng, A. P., & Deckwer, W. D. (1997). High concentration and productivity of 1,3-propanediol from continuous fermentation of glycerol by Klebsiella pneumoniae. Enzyme and Microbial Technology, 20(2), 82–86.
Przystalowska, H., Zeyland, J., Szymanowska-Powalowska, D., Szalata, M., Slomski, R., & Lipinski, D. (2015). 1,3-Propanediol production by new recombinant Escherichia coli containing genes from pathogenic bacteria. Microbiological Research, 171, 1–7.
Sen, B., Dabir, A. P., Lanjekar, V. B., & Ranade, D. R. (2015). Isolation and partial characterization of a new strain of Klebsiella pneumoniae capable of high 1,3 propanediol production from glycerol. Global Journal of Environmental Science and Management, 1(2), 99–108.
Vieira, P. B., Kilikian, B. V., Bastos, R. V., Perpetuo, E. A., & Nascimento, C. A. O. (2015). Process strategies for enhanced production of 1,3-propanediol by Lactobacillus reuteri using glycerol as a co-substrate. Biochemical Engineering Journal, 94, 30–38.
Zhang, G., Yang, G., Wang, W., Guo, Q., Li, Y., & Li, J. (2011). Influence of blocking of 2,3-butanediol production on glycerol metabolism for 1,3-PD production by Klebsiella pneumonia. Applied Biochemistry and Biotechnology, 168(1), 116–128.
Tong, I., Liao, H., & Cameron, D. (1991). 1,3-propanediol production by Escherichia coli expressing genes from Klebsiella pneumonia dha regulon. Applied and Environmental Microbiology, 57, 3541–3546.
Leja, K., Czaczyk, K., & Myszka, K. (2011). The use of microorganisms in 1,3-propanediol production. African Journal of Microbiology Research, 5(26), 4652–4658.
Zhu, M., Lawman, P., & Cameron, D. (2002). Improving 1, 3-propanediol production from glycerol in a metabolically engineered Escherichia coli by reducing accumulation of sn-glycerol-3-phosphate. Biotechnology Progress, 18, 694–699.
Zhuge, B., Zhang, C., Fang, H., Zhuge, J., & Permaul, K. (2010). Expression of 1,3-propanediol oxidoreductase and its isoenzyme in Klebsiella pneumoniae for bioconversion of glycerol into 1,3-propanediol. Applied Microbiology and Biotechnology, 87(6), 2177–2184.
Gonzalez-Pajuelo, M., Meynial-Salles, I., Mendes, F., Soucaille, P., & Vasconcelos, I. (2006). Microbial conversion of glycerol to 1,3-propanediol: physiological comparison of a natural producer, Clostridium butyricum VPI 3266, and an engineered strain, Clostridium acetobutylicum DG1(pSPD5). Applied and Environmental Microbiology, 72(1), 96–101.
Maervoet, V. E., De Maeseneire, S. L., Avci, F. G., Beauprez, J., Soetaert, W. K., & De Mey, M. (2016). High yield 1,3-propanediol production by rational engineering of the 3-hydroxypropionaldehyde bottleneck in Citrobacter werkmanii. Microbial Cell Factories, 15(1), 23.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Liu, Jz., Xu, W., Chistoserdov, A. et al. Glycerol Dehydratases: Biochemical Structures, Catalytic Mechanisms, and Industrial Applications in 1,3-Propanediol Production by Naturally Occurring and Genetically Engineered Bacterial Strains. Appl Biochem Biotechnol 179, 1073–1100 (2016). https://doi.org/10.1007/s12010-016-2051-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-016-2051-6