Abstract
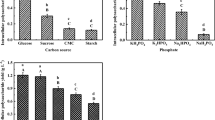
The present study describes the improved mycelia and exo-polymer production under control of Grifola frondosa morphology by changing the aeration rate and agitation intensity in a 25-L stirred fermentor. The aeration rate of 1.0 vvm yielded a highest mycelia biomass of 24.754 g/L with the lowest pellet percentage of 20.5 %. The maximum exo-polymer (2.324 g/L) was achieved at 0.75 vvm with mycelia polysaccharide production (0.321 g/g), whereas clumps and filaments dominated the ratios of 45.6 and 33.9 %, respectively. The change of aeration rate and agitation intensity had slight influence on the monosaccharide compositions in exo-polymers and significantly affected glucose and mannose contents in the mycelia polysaccharides. These findings will provide a clue for exploring the relationship between fermentation parameters, morphologies, and polysaccharide synthesis pathway of G. frondosa.




Similar content being viewed by others
References
De Silva, D. D., Rapior, S., Sudarman, E., Stadler, M., Xu, J. C., Alias, S. A., & Hyde, K. D. (2013). Bioactive metabolites from macrofungi: ethnopharmacology, biological activities and chemistry. Fungal Diversity, 62, 1–40.
Xu, X. Q., Shen, M. W., & Quan, L. L. (2015). Stimulatory agents simultaneously improving the production and antioxidant activity of polyphenols from Inonotus obliquus by submerged fermentation. Applied Biochemistry and Biotechnology, 176(5), 1237–1250.
Parenti, A., Muguerza, E., Iroz, A. R., Omarini, A., Conde, E., Alfaro, M., Castanera, R., Santoyo, F., Ramirez, L., & Pisabarro, A. G. (2013). Induction of laccase activity in the white rot fungus Pleurotus ostreatus using water polluted with wheat straw extracts. Bioresource Technology, 133, 142–149.
Silva, A. M., Miranda, A., Fernandes, E., Santos, S., Fraga, I., Santos, D. L., Dias, A. A., & Bezerra, R. M. (2013). Endopolysaccharides from Ganoderma resinaceum, Phlebia rufa, and Trametes versicolor affect differently the proliferation rate of HepG2 cells. Applied Biochemistry and Biotechnology, 169(6), 1919–1926.
Cui, J. D., & Zhang, Y. N. (2012). Evaluation of metal ions and surfactants effect on cell growth and exopolysaccharide production in two-stage submerged culture of Cordyceps militaris. Applied Biochemistry and Biotechnology, 168(6), 1394–1404.
van Dissel, D., Claessen, D., & van Wezel, G. (2014). Morphogenesis of Streptomyces in submerged cultures. Advances in Applied Microbiology, 89, 1–45.
Krull, R., Wucherpfennig, T., Esfandabadi, M. E., Walisko, R., Melzer, G., Hempel, D. C., Kampen, I., Kwade, A., & Wittmann, C. (2013). Characterization and control of fungal morphology for improved production performance in biotechnology. Journal of Biotechnology, 163, 112–123.
Papagianni, M., & Matter, M. (2006). Morphological development of Aspergillus niger in submerged citric acid fermentation as a function of the spore inoculum level. Application of neural network and cluster analysis for characterization of mycelial morphology. Microbial Cell Factories, 5, 3.
Gao, D. F., Zeng, J. J., Yu, X. C., Dong, T., & Chen, S. L. (2014). Improved lipid accumulation by morphology engineering of oleaginous fungus Mortierella isabellina. Biotechnology and Bioengineering, 111, 1758–1766.
Wucherpfennig, T., Kiep, K. A., Driouch, H., Wittmann, C., & Krull, R. (2010). Morphology and rheology in filamentous cultivations. Advances in Applied Microbiology, 72, 89–136.
Park, J. P., Kim, Y. M., Kim, S. W., & Hwan, H. J. (2002). Effect of aeration rate on the mycelial morphology and exo-biopolymer production in Cordyceps militaris. Process Biochemistry, 37, 1257–1262.
Fazenda, M. L., Harvey, L. M., & McNeil, B. (2010). Effects of dissolved oxygen on fungal morphology and process rheology during fed-batch processing of Ganoderma lucidum. Journal of Microbiology and Biotechnology, 20, 844–851.
Klaus, A., Kozarski, M., Vunduk, J., Todorovic, N., Jakovljevic, D., Zizak, Z., Pavlovic, V., Levic, S., Niksic, M., & Van Griensven, L. J. L. D. (2015). Biological potential of extracts of the wild edible Basidiomycete mushroom Grifola frondosa. Food Research International, 67, 272–283.
Sato, M., Tokuji, Y., Yoneyama, S., Fujii-Akiyama, K., Kinoshita, M., Chiji, H., & Ohnishi, M. (2013). Effect of dietary maitake (Grifola frondosa) mushrooms on plasma cholesterol and hepatic gene expression in cholesterol-fed mice. Journal of Oleo Science, 62, 1049–1058.
Tsao, Y. W., Kuan, Y. C., Wang, J. L., & Sheu, F. (2013). Characterization of a novel maitake (Grifola frondosa) protein that activates natural killer and dendritic cells and enhances antitumor immunity in mice. Journal of Agricultural and Food Chemistry, 61, 9828–9838.
Hsieh, C., Wang, W. H. L., Chen, C. C., Hsu, T. H., & Tseng, M. H. (2008). Effect of plant oil and surfactant on the production of mycelial biomass and polysaccharides in submerged culture of Grifola frondosa. Biochemical Engineering Journal, 38, 198–206.
Wu, C. Y., & Wu, T. X. (2015). Effect of the ingredients of Rhizoma gastrodiae on mycelial biomass and exopolysaccharide productions by submerged culture of Grifola frondosa. International Journal of Food Science and Technology, 50, 1726–1730.
Cui, F. J., Li, Y., Xu, Z. H., Xu, H. Y., Sun, K., & Tao, W. Y. (2006). Optimization of the medium composition for production of mycelial biomass and exo-polymer by Grifola frondosa GF9801 using response surface methodology. Bioresource Technology, 97, 1209–1216.
Cui, F. J., Tao, W. Y., Xu, Z. H., Guo, W. J., Xu, H. Y., Ao, Z. H., Jin, J., & Wei, Y. Q. (2007). Structural analysis of anti-tumor heteropolysaccharide GFPS1b from the cultured mycelia of Grifola frondosa GF9801. Bioresource Technology, 98, 395–401.
Cui, F. J., Zan, X. Y., Li, Y. H., Yang, Y., Sun, W. J., Zhou, Q., Yu, S. L., & Dong, Y. (2013). Purification and partial characterization of a novel anti-tumor glycoprotein from cultured mycelia of Grifola frondosa. International Journal of Biological Macromolecules, 62, 684–690.
Cui, F. J., Li, Y., Xu, Y. Y., Liu, Z. Q., Huang, D. M., Zhang, Z. C., & Tao, W. Y. (2007). Induction of apoptosis in SGC-7901 cells by polysaccharide-peptide GFPS1b from the cultured mycelia of Grifola frondosa GF9801. Toxicology in Vitro, 21, 417–427.
Riley, G. L., Tucker, K. G., Paul, G. C., & Thomas, C. R. (2000). Effect of biomass concentration and mycelial morphology on fermentation broth theology. Biotechnology and Bioengineering, 68, 160–172.
Sluiter A., Hames B., Ruiz R., Scarlata C., Sluiter J. & Templeton D. (2008) Determination of structural carbohydrates and lignin in biomass. National Renewable Energy Laboratory (NREL): laboratory analytical procedures (LAP) for standard biomass analysis.
Wucherpfennig, T., Hestler, T., & Krull, R. (2011). Morphology engineering—osmolality and its effect on Aspergillus niger morphology and productivity. Microbial Cell Factories, 10, 58.
Peberdy, J. F. (1994). Protein secretion in filamentous fungi trying to understand a highly productive black box. Trends in Biotechnology, 12, 50–57.
Punt, P. J., Veldhuisen, G., & van den Hondel, C. A. J. J. (1994). Protein targeting and secretion in filamentous fungi—a progress report. Antonie Van Leeuwenhoek, 65, 211–216.
Ahamed, A., & Vermette, P. (2009). Effect of culture medium composition on Trichoderma reesei’s morphology and cellulose production. Bioresource Technology, 100, 5979–5987.
Darah, I., Sumathi, G., Jain, K., & Lim, S. H. (2011). Influence of agitation speed on tannase production and morphology of Aspergillus niger FETL FT3 in submerged fermentation. Applied Biochemistry and Biotechnology, 165, 1682–1690.
Gibbs, P. A., & Seviour, R. J. (1996). Does the agitation rate and/or oxygen saturation influence exopolysaccharide production by Aureobasidium pullulans in batch culture? Applied Microbiology and Biotechnology, 46, 503–510.
Kim, S. S., Lee, J. S., Cho, J. Y., Kim, Y. E., & Hong, E. K. (2010). Process development for mycelia growth and polysaccharide production in Tricholoma matsutake liquid culture. Journal of Bioscience and Bioengineering, 109, 351–355.
Tepwong, P., Giri, A., & Ohshima, T. (2012). Effect of mycelial morphplogy on ergothineine production during liquid fermentation of Lentinula edodes. Mycoscience, 53, 102–112.
Lee, B. C., Bae, J. T., Pyo, H. B., & Choe, T. B. (2004). Submerged culture conditions for the production of mycelia biomass and exo-polysaccharides by the edible Basidiomycete Grifola frondosa. Enzyme and Microbial Technology, 35, 369–376.
Xu, C. P., Kim, S. W., Hwang, H. J., & Yun, J. W. (2006). Production of exopolysaccharides by submerged culture of an enthomopathogenic fungus, Paecilomyces tenuipes C 240 in stirred-tank and airlift reactors. Bioresource Technology, 97, 770–777.
Bellou, S., Makri, A., Triantaphyllidou, I. E., Papanikolaou, S., & Aggelis, G. (2014). Morphological and metabolic shifts of Yarrowia lipolytica induced by alteration of the dissolved oxygen concentration in the growth environment. Microbiology, 160, 807–817.
Dörmann, P., & Benning, C. (1996). Functional expression of uridine 5′-diphospho-glucose 4-epimerase from Arabidopsis thaliana in Saccharomyces cerevisiae and Escherichia coli. Archives of Biochemistry and Biophysics, 327, 27–34.
Harper, A. D., & Bar-Peled, M. (2002). Cloning and characterization of a novel Arabidopsis gene family, UXS, encoding soluble and putative membrane-bound UDP-glucuronic acid decarboxylase isoforms. Plant Physiology, 130, 2188–2198.
Lukowitz, W., NicHe, T. C., Meinke, D. W., Last, R. L., Conklin, P. L., & Somerville, C. R. (2001). Arabidopsis cyt1 mutants are deficient in a mannose-1-phosphate guanylyl transferase and point to a requirement of N-linked glycosylation for cellulose biosynthesis. Proceedings of the National Academy of Sciences, 5, 2262–2267.
Tang, Y. J., & Zhong, J. J. (2002). Exopolysaccharide biosynthesis and related enzyme activities of the medicinal fungus, Ganoderma lucidum, grown on lactose in a bioreactor. Biotechnology Letters, 24, 1023–1026.
Acknowledgments
This work was supported by funding from the Natural Science Foundation of China (NSFC 31101269), China Postdoctoral Science Special Foundation (2013T60648), 2012 Excellent Key Young Teachers Project of Jiangsu University, Science & Technology Platform Construction Program of Jiangxi Province, and A Project Funded by the Priority Academic Program Development of Jiangsu Higher Education Institutions.
Author information
Authors and Affiliations
Corresponding authors
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Fig. S1
Typical morphological changes of G. frondosa GF9801 in a 25-L stirred fermentor at different agitation intensities of 60 rpm (a), 90 rpm (b), 120 rpm (c), and 150 rpm (d) during the 7-day fermentation period at 0.5 vvm and 28 °C. The bar is 1 mm. (DOCX 11,748 kb)
Fig. S2
Typical morphological changes of G. frondosa GF9801 in a 25-L stirred fermentor at different aeration rates of 0.25 vvm (a), 0.5 vvm (b), 0.75 vvm (c), and 1.0 vvm (d) during the 7-day fermentation period at 90 rpm and 28 °C. Bar = 1 mm. (DOCX 6816 kb)
Table S1
Morphological parameters of pellets/mycelial aggregates of G. frondosa at different agitation intensities (DOCX 35 kb)
Table S2
Morphological parameters of pellets/mycelial aggregates of G. frondosa at different aeration rates. (DOCX 35 kb)
Table S3
Effects of aeration rates and agitation intensities on monosaccharide composition of mycelia polysaccharide produced by G. frondosa GF 9801. (DOCX 36 kb)
Rights and permissions
About this article
Cite this article
Cui, FJ., Chen, XX., Liu, WM. et al. Control of Grifola frondosa Morphology by Agitation and Aeration for Improving Mycelia and Exo-Polymer Production. Appl Biochem Biotechnol 179, 459–473 (2016). https://doi.org/10.1007/s12010-016-2006-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-016-2006-y