Abstract
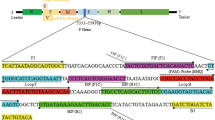
Biological therapeutic products such as recombinant hepatitis B virus (HBV) vaccine, produced by microbial fermentation in complex media, should be evaluated for host cell DNA contamination in purification steps. Eliminating these contaminations increases the efficacy of the vaccine and decreases its side effects. The objective of the present study is to trace the residual host cell DNA (HCD) in recombinant HBV vaccine by developing a TaqMan Real-Time PCR method which is more sensitive, specific, and reproducible than traditional methods such as Picogreen analysis and Threshold DNA assay. Primers and a probe were designed for the most highly conserved regions of Pichia pastoris genome. To determine the specificity of the assay, in addition to performing a BLAST for the primers and the probe in NCBI nucleotide database, 20 different human genomes and 8 bacterial and viral genomes were used. Moreover, serial dilutions of plasmids, from 102 to 107 copies/μL (from 0.00064 to 6.4 pg/μL), were prepared to find the sensitivity and the limit of detection (LOD) of the assay. Using 28 different genome samples, the specificity of the assay was determined to be 100 %. In addition, the sensitivity and LOD of the method was 0.39 × 10−5 pg/μL. Moreover, the reproducibility of the assay based on intra- and inter-assay was 1.03 and 1.06 %, respectively. Considering the suitable specificity and sensitivity, ease of use, relatively low cost, and rapidity of the assay, it can be a reproducible and sensitive method to examine recombinant vaccines for P. pastoris residual DNA.


Similar content being viewed by others
References
Lok, A. S., & McMahon, B. J. (2009). Chronic hepatitis B: update 2009. Hepatology, 50(3), 661–662.
Romano, L., et al. (2013). Hepatitis B virus infection among first-time blood donors in Italy: prevalence and correlates between serological patterns and occult infection. Blood Transfusion, 11(2), 281–288.
Wallace, J., et al. (2012). Challenges to the effective delivery of health care to people with chronic hepatitis B in Australia. Sexual Health, 9(2), 131–137.
Kao, J. H., & Chen, D. S. (2002). Global control of hepatitis B virus infection. Lancet Infectious Diseases, 2(7), 395–403.
Liang, X., et al. (2009). Epidemiological serosurvey of hepatitis B in China—declining HBV prevalence due to hepatitis B vaccination. Vaccine, 27(47), 6550–6557.
Bo, H., et al. (2005). Expression of hepatitis B virus S gene in Pichia pastoris and application of the product for detection of anti-HBs antibody. Journal of Biochemistry and Molecular Biology, 38(6), 683–689.
Salahuddin, S. Z., et al. (2007). The simultaneous presence and expression of human hepatitis C virus (HCV), human herpesvirus-6 (HHV-6), and human immunodeficiency virus-1 (HIV-1) in a single human T-cell. Virology Journal, 4, 106.
Beldarraín Iznaga, A., et al. (2007). DNA removal from a purification process of recombinant hepatitis B surface antigen. Electronic Journal of Biotechnology, 10(1), 37–47.
Yang, H. and J. Zhang, A Bayesian approach to residual host cell DNA safety assessment. PDA J Pharm Sci Technol, 2016.
Hardy, E., et al. (2000). Large-scale production of recombinant hepatitis B surface antigen from Pichia pastoris. Journal of Biotechnology, 77(2–3), 157–167.
Ikeda, Y., Iwakiri, S., & Yoshimori, T. (2009). Development and characterization of a novel host cell DNA assay using ultra-sensitive fluorescent nucleic acid stain “PicoGreen”. Journal of Pharmaceutical and Biomedical Analysis, 49(4), 997–1002.
Pripuzova, N., et al. (2012). Development of real-time PCR array for simultaneous detection of eight human blood-borne viral pathogens. PloS One, 7(8), e43246.
Alizadeh, Z., Milani, S., & Sharifi, Z. (2014). Occult hepatitis B virus infection among Iranian blood donors: a preliminary study. Archives of Iranian Medicine, 17(2), 106–107.
Heiat, M., Ranjbar, R., & Alavian, S. M. (2014). Classical and modern approaches used for viral hepatitis diagnosis. Hepatitis Monthly, 14(4), e17632.
Lee, D. H., et al. (2010). Quantitative detection of residual E. coli host cell DNA by real-time PCR. Journal of Microbiology and Biotechnology, 20(10), 1463–1470.
Paryan, M., et al. Simultaneous detection and genotype determination of HSV 1 and 2 by real-time PCR using melting curve analysis and a unique pair of primers. Appl Immunohistochem Mol Morphol, 2015
Riahi, F., et al. (2015). Evaluation of point mutation detection in Mycobacterium tuberculosis with isoniazid resistance using real-time PCR and TaqMan probe assay. Applied Biochemistry and Biotechnology, 175(5), 2447–2455.
Paryan, M., et al. (2012). Design and development of an in-house multiplex RT-PCR assay for simultaneous detection of HIV-1 and HCV in plasma samples. Iran Journal Microbiology, 4(1), 8–14.
Andersen, C. B., et al. (2006). Equal performance of TaqMan, MGB, molecular beacon, and SYBR green-based detection assays in detection and quantification of roundup ready soybean. Journal of Agricultural and Food Chemistry, 54(26), 9658–9663.
Lahijani, R., et al. (1998). Quantitation of host cell DNA contaminate in pharmaceutical-grade plasmid DNA using competitive polymerase chain reaction and enzyme-linked immunosorbent assay. Human Gene Therapy, 9(8), 1173–1180.
Wolter, T., & Richter, A. (2005). Assays for controlling host-cell impurities in biopharmaceuticals. BioProcess International, 3(2), 40–46.
Ji, X., Lee, K., & DiPaolo, B. (2002). High-sensitivity hybridization assay for quantitation of residual E. coli DNA. Biotechniques, 32(5), 1162–1167.
Wang, K. Y., et al. (2006). 16S rRNA gene probe quantitates residual host cell DNA in pharmaceutical-grade plasmid DNA. Vaccine, 24(14), 2656–2661.
Acknowledgments
The authors would like to thank Ms. Arlene Haghverdian for kindly final revision of the manuscript. This work was funded by Pasteur Institute of Iran (Grant No: 718) and Iran National Science Foundation (INSF, Grant No: 93010904), Tehran, Iran. The authors appreciate Pasteur Institute of Iran, Tehran, Iran, for providing technical support.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Paryan, M., Khodayar, M., Kia, V. et al. Development of an in-House TaqMan Real-Time PCR-Based Method to Detect Residual Host Cell DNA in HBV Vaccine. Appl Biochem Biotechnol 179, 375–382 (2016). https://doi.org/10.1007/s12010-016-2000-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-016-2000-4