Abstract
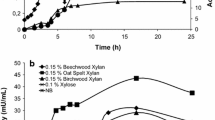
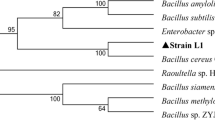
A low molecular weight xylanase from Bacillus strain CSB40, isolated from traditional Korean food and produced in beechwood xylan, was biochemically and thermodynamically characterized. It was purified 8.12-fold with a 15.88 % yield using DEAE sepharose fast flow, and it was determined to have a mass of ∼27 kDa via sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and xylan zymography. The purified xylanase was optimally active at 50 °C and pH 6 and stable over a wide range of pH (4.5–12.5). The N-terminal amino acid sequence of xylanase was GIQQGDDGKL. The activation energy for beechwood xylan hydrolysis was 29.39 kJmol−1 with k cat value of 927.582 × 102 s−1. K m and V max were 0.080 mg/ml and 794.63 mmol min−1 mg−1. The analysis of other thermodynamic parameters like ∆H, ∆G, ∆S, Q10, ∆GE-S, and ∆GE-T also supported the spontaneous formation of products, greater hydrolytic efficiency, and feasibility of enzymatic reaction, which also ratifies the novelty of this xylanase. The enzyme was strongly activated by Zn2+ and inhibited by Cu2+. The principal hydrolyzed end-products of this xylanase are xylobiose, xylotriose, and xylotetrose, which can be used in the pharmaceutical industry and as prebiotic in food.






Similar content being viewed by others
References
Luo, L., Cai, J., Wang, C., Lin, J., Du, X., Zhou, A., & Xiang, M. (2015). Purification and characterization of an alkaliphilic endo-xylanase from Streptomyces althioticusLMZM and utilization in the pulp paper industry. Journal of Chemical Technology & Biotechnology. doi:10.1002/jctb.4690.
Beg, Q. K., Kapoor, M., Mahajan, L., & Hoondal, G. S. (2001). Microbial xylanases and their industrial applications: a review. Applied Microbiology and Biotechnology, 56(3–4), 326–338.
Collins, T., Gerday, C., & Feller, G. (2005). Xylanases, xylanase families and extremophilic xylanases. FEMS Microbiology Reviews, 29(1), 3–23.
Liu, W., Zhu, W., Lu, Y., Kong, J., & Ma, G. (1998). Production, partial purification and characterization of xylanase from Trichosporon cutaneum SL409. Process Biochemistry, 33(3), 331–336.
Vohra, A., & Satyanarayana, T. (2012). Probiotic yeasts. In T. Satyanarayana, B. N. Johri, & A. Prakash (Eds.), Microorganisms in sustainable agriculture and biotechnology (pp. 411–434). Springer: New York.
Rahman, M. A., Choi, Y. H., Pradeep, G. C., Choi, Y. S., Choi, E. J., Cho, S. S., & Yoo, J. C. (2014). A novel low molecular weight endo-xylanase from Streptomyces sp. CS628 cultivated in wheat bran. Applied Biochemistry and Biotechnology, 173(6), 1469–1480.
Kumar, V., & Satyanarayana, T. (2013). Biochemical and thermodynamic characteristics of thermo-alkali-stable xylanase from a novel polyextremophilic Bacillus halodurans TSEV1. Extremophiles, 17(5), 797–808.
Mandal, A., Kar, S., Das Mohapatra, P. K., Maity, C., Pati, B. R., & Mondal, K. C. (2011). Purification and characterization of an endoxylanase from the culture broth of Bacillus cereus BSA11. Applied Biochemistry and Biotechnology, 47(3), 250–255.
Kiddinamoorthy, J., Anceno, A. J., Haki, G. D., & Rakshit, S. K. (2008). Production, purification and characterization of Bacillus sp. GRE7 xylanase and its application in eucalyptus Kraft pulp biobleaching. World Journal of Microbiology and Biotechnology, 24(5), 605–612.
Sá-Pereira, P., Duarte, J., & Costa-Ferreira, M. (2000). Electroelution as a simple and fast protein purification method: isolation of an extracellular xylanase from Bacillus sp. CCMI 966. Enzyme and Microbial Technology, 27(1–2), 95–99.
Lapidot, A., Mechaly, A., & Shoham, Y. (1996). Overexpression and single-step purification of a thermostable xylanase from Bacillus stearothermophilus T-6. Journal of Biotechnology, 51(3), 259–264.
Archana, A., & Satyanarayana, T. (2003). Purification and characterization of a cellulase-free xylanase of a moderate thermophile Bacillus licheniformis A99. World Journal of Microbiology and Biotechnology, 19(1), 53–57.
Anand, A., Kumar, V., & Satyanarayana, T. (2013). Characteristics of thermostable endoxylanase and β-xylosidase of the extremely thermophilic bacterium Geobacillus thermodenitrificans TSAA1 and its applicability in generating xylooligosaccharides and xylose from agro-residues. Extremophiles, 17(3), 357–366.
Mamo, G., Hatti-Kaul, R., & Mattiasson, B. (2006). A thermostable alkaline active endo-β-1-4-xylanase from Bacillus halodurans S7: purification and characterization. Enzyme and Microbial Technology, 39(7), 1492–1498.
Breccia, J. D., Siñeriz, F., Baigori, M. D., Castro, G. R., & Hatti-Kaul, R. (1998). Purification and characterization of a thermostable xylanase from Bacillus amyloliquefaciens. Enzyme and Microbial Technology, 22(1), 42–49.
Vázquez, M. J., Alonso, J. L., Domínguez, H., & Parajó, J. C. (2001). Xylooligosaccharides: manufacture and applications. Trends in Food Science and Technology, 11(11), 387–393.
Bradford, M. M. (1976). A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Analytical Biochemistry, 72, 248–254.
Miller, G. L. (1959). Use of dinitrosalicylic acid reagent for determination of reducing sugar. Analytical Chemistry, 31, 426–428.
Laemmli, U. K. (1970). Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature, 227(5259), 680–685.
Sriyapai, T., Somyoonsap, P., Matsui, K., Kawai, F., & Chansiri, K. (2011). Cloning of a thermostable xylanase from Actinomadura sp. S14 and its expression in Escherichia coli and Pichia pastoris. Journal of Bioscience and Bioengineering, 111(5), 528–536.
Eyring, H., & Stearn, A. E. (1939). The application of the theory of absolute reaction rates to proteins. Chemical Reviews, 24(2), 253–270.
Dixon, M., & Webb, E. C. (1958). Enzyme kinetics (Vol. 182). New York: Academic Press.
Kamble, R. D., & Jadhav, A. R. (2012). Isolation, purification, and characterization of xylanase produced by a new species of Bacillus in solid state fermentation. International Journal of Microbiology, 2012, 683193.
Bataillon, M., Nunes Cardinali, A. P., Castillon, N., & Duchiron, F. (2000). Purification and characterization of a moderately thermostable xylanase from Bacillus sp. strain SPS-0. Enzyme and Microbial Technology, 26(2–4), 187–192.
Nakamura, S., Wakabayashi, K., Nakai, R., Aono, R., & Horikoshi, K. (1993). Production of alkaline xylanase by a newly isolated alkaliphilic Bacillus sp. strain 41M-l. World Journal of Microbiology and Biotechnology, 9(2), 221–224.
Verma, D., & Satyanarayana, T. (2012). Cloning, expression and applicability of thermo-alkali-stable xylanase of Geobacillus thermoleovorans in generating xylooligosaccharides from agro-residues. Bioresource Technology, 107, 333–338.
Nanmori, T., Watanabe, T., Shinke, R., Kohno, A., & Kawamura, Y. (1990). Purification and properties of thermostable xylanase and beta-xylosidase produced by a newly isolated Bacillus stearothermophilus strain. Journal of Bacteriology, 172(12), 6669–6672.
Rajaram, S., & Varma, A. (1990). Production and characterization of xylanase from Bacillus thermoalkalophilus grown on agricultural wastes. Applied Microbiology and Biotechnology, 34(1), 141–144.
Heck, J. X., Flôres, S. H., Hertz, P. F., & Ayub, M. A. Z. (2005). Optimization of cellulase-free xylanase activity produced by Bacillus coagulans BL69 in solid-state cultivation. Process Biochemistry, 40(1), 107–112.
Li, C., Yuzhi, H., Zongze, S., Ling, L., Xiaoluo, H., Pengfu, L., & Ziduo, L. (2009). Novel alkali-stable, cellulase-free xylanase from deep-sea Kocuria sp. Mn22. Journal of Microbiology and Biotechnology, 19(9), 873–880.
Raghukumar, C., Muraleedharan, U., Gaud, V. R., & Mishra, R. (2004). Xylanases of marine fungi of potential use for biobleaching of paper pulp. Journal of Industrial Microbiology and Biotechnology, 31(9), 433–441.
Nakamura, S., Wakabayashi, K., Nakai, R., Aono, R., & Horikoshi, K. (1993). Purification and some properties of an alkaline xylanase from alkaliphilic Bacillus sp. strain 41M-1. Applied Environmental Microbiology, 59, 2311–2316.
Bokhari, S. A. I., Latif, F., & Rajoka, M. I. (2009). Purification and characterization of xylanases from Thermomyces lanuginosus and its mutant derivative possessing novel kinetic and thermodynamic properties. World Journal of Microbiology and Biotechnology, 25(3), 493–502.
Kar, S., Sona, G. S., Das, A., Jana, A., Maity, C., Mandal, A., & Mondal, K. C. (2013). Process optimization of xylanase production using cheap solid substrate by Trichoderma reesei SAF3 and study on the alteration of behavioral properties of enzyme obtained from SSF and SmF. Bioprocess and Biosystems Engineering, 36(1), 57–68.
Afzal, A. J., Ali, S., Latif, F., Rajoka, M. I., & Siddiqui, K. S. (2005). Innovative kinetic and thermodynamic analysis of a purified superactive xylanase from Scopulariopsis sp. Applied Biochemistry and Biotechnology, 120(1), 51–70.
Ul Haq, I., Hussain, Z., Khan, M. A., Muneer, B., Afzal, S., Majeed, S., & Akram, F. (2012). Kinetic and thermodynamic study of cloned thermostable endo-1,4-β- xylanase from Thermotoga petrophila in mesophilic host. Molecular Biology Reports, 39(7), 7251–7261.
Acknowledgments
This work was supported by research fund from Chosun University, 2015.
Author information
Authors and Affiliations
Corresponding author
Additional information
Sandesh Panthi and Yoon Seok Choi contributed equally to this work.
Rights and permissions
About this article
Cite this article
Panthi, S., Choi, Y.S., Choi, Y.H. et al. Biochemical and Thermodynamic Characterization of a Novel, Low Molecular Weight Xylanase from Bacillus Methylotrophicus CSB40 Isolated from Traditional Korean Food. Appl Biochem Biotechnol 179, 126–142 (2016). https://doi.org/10.1007/s12010-016-1983-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-016-1983-1