Abstract
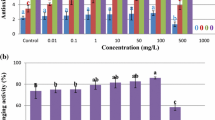
Fagonia indica, a very important anticancer plant, has been less explored for its in vitro potential. This is the first report on thidiazuron (TDZ)-mediated callogenesis and elicitation of commercially important phenolic compounds. Among the five different plant growth regulators tested, TDZ induced comparatively higher fresh biomass, 51.0 g/100 mL and 40.50 g/100 mL for stem and leaf explants, respectively, after 6 weeks of culture time. Maximum total phenolic content (202.8 μg gallic acid equivalent [GAE]/mL for stem-derived callus and 161.3 μg GAE/mL for leaf-derived callus) and total flavonoid content (191.03 μg quercetin equivalent [QE]/mL for stem-derived callus and 164.83 μg QE/mL for leaf-derived callus) were observed in the optimized callus cultures. The high-performance liquid chromatography (HPLC) data indicated higher amounts of commercially important anticancer secondary metabolites such as gallic acid (125.10 ± 5.01 μg/mL), myricetin (32.5 ± 2.05 μg/mL), caffeic acid (12.5 ± 0.52 μg/mL), catechin (9.4 ± 1.2 μg/mL), and apigenin (3.8 ± 0.45 μg/mL). Owing to the greater phenolic content, a better 2-2-diphenyl-1-picrylhydrazyl (DPPH) radical-scavenging activity (69.45 % for stem explant and 63.68 % for leaf explant) was observed in optimized calluses. The unusually higher biomass and the enhanced amount of phenolic compounds as a result of lower amounts of TDZ highlight the importance of this multipotent hormone as elicitor in callus cultures of F. indica.



Similar content being viewed by others
References
Cancer 2015. Available from: http://www.who.int/mediacentre/factsheets/fs297/en/. Accessed 28 Jun 2015.
Roleira, F. M., Tavares-da-Silva, E. J., Varela, C. L., Costa, S. C., Silva, T., Garrido, J., & Borges, F. (2015). Plant derived and dietary phenolic antioxidants: anticancer properties. Food Chemistry, 183, 235–258.
Lam, M., Carmichael, A. R., & Griffiths, H. R. (2012). An aqueous extract of Fagonia cretica induces DNA damage, cell cycle arrest and apoptosis in breast cancer cells via FOXO3a and p53 expression. PloS One, 7, e40152.
Waheed, A., Barker, J., Barton, S. J., Owen, C. P., Ahmed, S., & Carew, M. A. (2012). A novel steroidal saponin glycoside from Fagonia indica induces cell-selective apoptosis or necrosis in cancer cells. European Journal of Pharmaceutical Sciences, 47, 464–473.
Saeed, M. A. (1969) Hamdard pharmacopoeia of Eastern medicine. pp. 41–43. Hamdard Academy, Karachi, Pakistan.
Pareek, A., Godavarthi, A., Issarani, R., & Nagori, B. P. (2013). Antioxidant and hepatoprotective activity of Fagonia schweinfurthii (Hadidi) Hadidi extract in carbon tetrachloride induced hepatotoxicity in HepG2 cell line and rats. Journal of Ethnopharmacology, 150, 973–981.
Saleem, S., Jafri, L., ul Haq, I., Chang, L. C., Calderwood, D., Green, B. D., & Mirza, B. (2014). Plants Fagonia cretica L. and Hedera nepalensis K. Koch contain natural compounds with potent dipeptidyl peptidase-4 (DPP-4) inhibitory activity. Journal of Ethnopharmacology, 156, 26–32.
Alqasoumi, S. I., Yusufoglu, H. S., & Alam, A. (2011). Anti-inflammatory and wound healing activity of Fagonia schweinfurthii alcoholic extract herbal gel on albino rats. African Journal of Pharmacy and Pharmacology, 5, 1996–2001.
Rasool, B. K. A., Shehab, N. G., Khan, S. A., & Bayoumi, F. A. (2014). A new natural gel of Fagonia indica Burm f. extract for the treatment of burn on rats. Pakistan Journal of Pharmaceutical Sciences, 27, 73–81.
Bagban, I. M., Roy, S. P., Chaudhary, A., Das, S. K., Gohil, K. J., & Bhandari, K. K. (2012). Hepatoprotective activity of the methanolic extract of Fagonia indica Burm in carbon tetra chloride induced hepatotoxicity in albino rats. Asian Pacific Journal of Tropical Biomedicine, 2, S1457–S1460.
Ansari, A. A., & Kenne, L. (1984). Hederagenin, ursolic acid, and pinatol from Fagonia indica. Journal of Natural Products, 47, 186–187.
Shaker, K. H., Bernhardt, M., Elgamal, M. H. A., & Seifert, K. (2000). Sulfonated triterpenoid saponins from Fagonia indica. Zeitschrift Fur Naturforschung Section C-a Journal of Biosciences, 55, 520–523.
Khan, M. A., Abbasi, B., Ali, H., Ali, M., Adil, M., & Hussain, I. (2015). Temporal variations in metabolite profiles at different growth phases during somatic embryogenesis of Silybum marianum L. Plant Cell Tissue Organic Culture, 120, 127–139.
Matkowski, A. (2008). Plant in vitro culture for the production of antioxidants—a review. Biotechnology Advances, 26, 548–560.
Davies, K. M., & Deroles, S. C. (2014). Prospects for the use of plant cell cultures in food biotechnology. Current Opinion in Biotechnology, 26, 133–140.
Bahri-Sahloul, R., Ben Fredj, R., Boughalleb, N., Shriaa, J., Saguem, S., Hilbert, J.-L., Trotin, F., Ammar, S., Bouzid, S., & Harzallah-Skhiri, F. (2014). Phenolic composition and antioxidant and antimicrobial activities of extracts obtained from Crataegus azarolus L. var. aronia (Willd.) Batt. ovaries calli. Journal of Botany, 2014, 11.
Slinkard, K., & Singleton, V. L. (1977). Total phenol analysis: automation and comparison with manual methods. American Journal of Enology and Viticulture, 28, 49–55.
Chang, C.-C., Yang, M.-H., Wen, H.-M. and Chern, J.-C. (2002). Estimation of total flavonoid content in propolis by two complementary colorimetric methods. Journal of Food and Drug Analysis. 10.
Shah, N. A., Khan, M. R., Naz, K., & Khan, M. A. (2014). Antioxidant potential, DNA protection, and HPLC-DAD analysis of neglected medicinal Jurinea dolomiaea roots. BioMed Research International, 2014, 726241.
Khan, M. A., Abbasi, B. H., Ahmed, N., & Ali, H. (2013). Effects of light regimes on in vitro seed germination and silymarin content in Silybum marianum. Industrial Crops and Products, 46, 105–110.
Amarowicz, R., Pegg, R. B., Rahimi-Moghaddam, P., Barl, B., & Weil, J. A. (2004). Free-radical scavenging capacity and antioxidant activity of selected plant species from the Canadian prairies. Food Chemistry, 84, 551–562.
Murthy, B., Murch, S., & Saxena, P. K. (1998). Thidiazuron: a potent regulator of in vitro plant morphogenesis. In Vitro Cellular & Developmental Biology-Plant, 34, 267–275.
Thomas, J. C., & Katterman, F. R. (1986). Cytokinin activity induced by thidiazuron. Plant Physiology, 81, 681–683.
Eman, A. A., Gehan, H. A., Yassin, M., & Mohamed, S. (2010). Chemical composition and antibacterial activity studies on callus of Fagonia arabica L. Academia Arena, 2, 91–106.
Ebrahimi, M. A., & Payan, A. (2013). Induction of callus and somatic embryogenesis from cotyledon explants of Fagonia indica Burm. Journal of Medicinal Plants and By-Products, 2, 209–214.
Palmer, C. D., & Keller, W. (2011). Plant regeneration using immature zygotic embryos of Tribulus terrestris. Plant Cell, Tissue and Organ Culture, 105, 121–127.
Mathur, S., & Shekhawat, G. S. (2013). Establishment and characterization of Stevia rebaudiana (Bertoni) cell suspension culture: an in vitro approach for production of stevioside. Acta Physiologiae Plantarum, 35, 931–939.
Ali, M., & Abbasi, B. H. (2014). Thidiazuron-induced changes in biomass parameters, total phenolic content, and antioxidant activity in callus cultures of Artemisia absinthium L. Applied Biochemistry and Biotechnology, 172, 2363–2376.
Nikam, T., Ebrahimi, M. A., & Patil, V. (2009). Embryogenic callus culture of Tribulus terrestris L. a potential source of harmaline, harmine and diosgenin. Plant Biotechnology Reports, 3, 243–250.
Rice-Evans, C., Miller, N., & Paganga, G. (1997). Antioxidant properties of phenolic compounds. Trends in Plant Science, 2, 152–159.
Pourebad, N., Motafakkerazad, R., Kosari-Nasab, M., Farsad Akhtar, N., & Movafeghi, A. (2015). The influence of TDZ concentrations on in vitro growth and production of secondary metabolites by the shoot and callus culture of Lallemantia iberica. Plant Cell, Tissue and Organ Culture, 122, 331–339.
Bhargava, A., Clabaugh, I., To, J. P., Maxwell, B. B., Chiang, Y.-H., Schaller, G. E., Loraine, A., & Kieber, J. J. (2013). Identification of cytokinin-responsive genes using microarray meta-analysis and RNA-Seq in Arabidopsis. Plant Physiology, 162, 272–294.
Karam, N. S., Jawad, F. M., Arikat, N. A., & Shibl, R. A. (2003). Growth and rosmarinic acid accumulation in callus, cell suspension, and root cultures of wild Salvia fruticosa. Plant Cell, Tissue and Organ Culture, 73, 117–121.
Shibli, R., Smith, M. A. L., & Kushad, M. (1997). Headspace ethylene accumulation effects on secondary metabolite production in Vaccinium pahalae cell culture. Plant Growth Regulation, 23, 201–205.
Haslam, E., & Cai, Y. (1994). Plant polyphenols (vegetable tannins): gallic acid metabolism. Natural Product Reports, 11, 41–66.
Mayr, C., Wagner, A., Neureiter, D., Pichler, M., Jakab, M., Illig, R., Berr, F., & Kiesslich, T. (2015). The green tea catechin epigallocatechin gallate induces cell cycle arrest and shows potential synergism with cisplatin in biliary tract cancer cells. BMC Complementary and Alternative Medicine, 15, 194.
Nabavi, S., Habtemariam, S., Daglia, M. and Nabavi, S. (2015) Apigenin and breast cancers: from chemistry to medicine. Anti-Cancer Agents in Medicinal Chemistry.
Rosendahl, A. H., Perks, C. M., Zeng, L., Markkula, A., Simonsson, M., Rose, C., Ingvar, C., Holly, J. M. P., & Jernström, H. (2015). Caffeine and caffeic acid inhibit growth and modify estrogen receptor and insulin-like growth factor I receptor levels in human breast cancer. Clinical Cancer Research, 21, 1877–1887.
Subramanian, A. P., John, A. A., Vellayappan, M. V., Balaji, A., Jaganathan, S. K., Supriyanto, E., & Yusof, M. (2015). Gallic acid: prospects and molecular mechanisms of its anticancer activity. RSC Advances, 5, 35608–35621.
Yi, J. L., Shi, S., Shen, Y. L., Wang, L., Chen, H. Y., Zhu, J., & Ding, Y. (2015). Myricetin and methyl eugenol combination enhances the anticancer activity, cell cycle arrest and apoptosis induction of cis-platin against HeLa cervical cancer cell lines. International Journal of Clinical and Experimental Pathology, 8, 1116–1127.
Chen, C.-H., Chan, H.-C., Chu, Y.-T., Ho, H.-Y., Chen, P.-Y., Lee, T.-H., & Lee, C.-K. (2009). Antioxidant activity of some plant extracts towards xanthine oxidase, lipoxygenase and tyrosinase. Molecules, 14, 2947.
Abbasi, B., Khan, M., Mahmood, T., Ahmad, M., Chaudhary, M., & Khan, M. (2010). Shoot regeneration and free-radical scavenging activity in Silybum marianum L. Plant Cell, Tissue and Organ Culture, 101, 371–376.
Camm, E. L., & Towers, G. N. (1973). Phenylalanine ammonia lyase. Phytochemistry, 12, 961–973.
Schuster, B., & Retey, J. (1995). The mechanism of action of phenylalanine ammonia-lyase: the role of prosthetic dehydroalanine. Proceedings of the National Academy of Sciences, 92, 8433–8437.
Nagai, N., Kitauchi, F., Okamoto, K., Kanda, T., Shimosaka, M., & Okazaki, M. (1994). A transient increase of phenylalanine ammonia-lyase transcript in kinetin-treated tobacco callus. Bioscience, Biotechnology, and Biochemistry, 58, 558–559.
Acknowledgments
Tariq Khan acknowledges the indigenous PhD fellowship program of the Higher Education Commission (HEC), Pakistan. Bilal Haider Abbasi acknowledges the financial support from the Pakistan Academy of Sciences (PAS), Pakistan.
Authors’ Contributions
TK did the research work and wrote the manuscript. BHA conceived the idea and supervised the work. MAK and BHA analyzed the data. BHA and ZKS critically reviewed the manuscript and added to its technical part.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no competing interest.
Rights and permissions
About this article
Cite this article
Khan, T., Abbasi, B.H., Khan, M.A. et al. Differential Effects of Thidiazuron on Production of Anticancer Phenolic Compounds in Callus Cultures of Fagonia indica . Appl Biochem Biotechnol 179, 46–58 (2016). https://doi.org/10.1007/s12010-016-1978-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-016-1978-y