Abstract
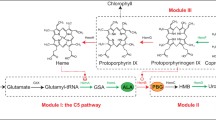
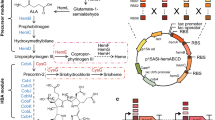
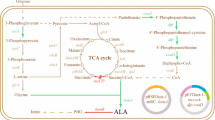
5-Aminolevulinic acid (ALA) is a nonprotein amino acid that has been widely used in many fields. In this study, we developed a new process for ALA production by optimizing the in vivo heme biosynthesis pathway and the iron concentration during cultivation. With the addition of iron, co-overexpression of the heme synthesis pathway genes hemA, hemL, hemF, and hemD significantly increased the accumulation of ALA and cell biomass. Further experiments demonstrated that the increased ALA accumulation resulted from moderate repression of ALA dehydratase (encoded by hemB), which was caused by hemF overexpression. After the addition of an optimized concentration (7.5 mg/L) of iron, ALA production by the recombinant Escherichia coli LADF-6 strain that overexpressed hemA, hemL, hemD, and hemF increased to 2840 mg/L in flask cultures. After applying a batch fermentation strategy, the ALA concentration increased to 4.05 g/L, with a productivity of 0.127 g/L·h. The results showed that the moderate repression of the in vivo heme pathway enzyme ALA dehydratase and the simultaneous optimization of the in vitro iron ion concentration served to increase the production of ALA and cell biomass.





Similar content being viewed by others
References
Kang, Z., Zhang, J., Zhou, J., Qi, Q., Du, G., & Chen, J. (2012). Recent advances in microbial production of δ-aminolevulinic acid and vitamin B12. Biotechnology Advances, 30, 1533–1542.
Beale, S. I. (1990). Biosynthesis of the tetrapyrrole pigment precursor, delta-aminolevulinic acid, from glutamate. Plant Physiology, 93, 1273–1279.
Hunter, G., & Ferreira, G. (2009). 5-Aminolevulinate synthase: catalysis of the first step of heme biosynthesis. Cell and Molecular Biology (Noisy-le-grand), 55, 102–110.
Sasaki, K., Watanabe, M., Tanaka, T., & Tanaka, T. (2002). Biosynthesis, biotechnological production and applications of 5-aminolevulinic acid. Applied Microbiology and Biotechnology, 58, 23–29.
Kang, Z., Wang, Y., Gu, P., Wang, Q., & Qi, Q. (2011). Engineering Escherichia coli for efficient production of 5-aminolevulinic acid from glucose. Metabolic Engineering, 13, 492–498.
Luer, C., Schauer, S., Mobius, K., Schulze, J., Schubert, W. D., Heinz, D. W., Jahn, D., & Moser, J. (2005). Complex formation between glutamyl-tRNA reductase and glutamate-1-semialdehyde 2,1-aminomutase in Escherichia coli during the initial reactions of porphyrin biosynthesis. The Journal of Biological Chemistry, 280, 18568–18572.
Bhowmick, R., & Girotti, A. W. (2010). Cytoprotective induction of nitric oxide synthase in a cellular model of 5-aminolevulinic acid-based photodynamic therapy. Free Radical Biology and Medicine, 48, 1296–1301.
Mikolajewska, P., Donnelly, R. F., Garland, M. J., Morrow, D. I., Singh, T. R., Iani, V., Moan, J., & Juzeniene, A. (2010). Microneedle pre-treatment of human skin improves 5-aminolevulininc acid (ALA)- and 5-aminolevulinic acid methyl ester (MAL)-induced PpIX production for topical photodynamic therapy without increase in pain or erythema. Pharmaceutical Research, 27, 2213–2220.
Sasikala, C., Ramana, C. V., & Rao, P. R. (1994). 5-Aminolevulinic acid: a potential herbicide/insecticide from microorganisms. Biotechnology Progress, 10, 451–459.
Takeya, H., Tanaka, T., Hotta, T., & Sasaki, K. (1997). Production methods and applications of 5-aminolevulinic acid. Porphyrins, 6, 127–135.
Lin, J., Fu, W., & Cen, P. (2009). Characterization of 5-aminolevulinate synthase from Agrobacterium radiobacter, screening new inhibitors for 5-aminolevulinate dehydratase from Escherichia coli and their potential use for high 5-aminolevulinate production. Bioresource Technology, 100, 2293–2297.
Neidle, E. L., & Kaplan, S. (1993). Expression of the Rhodobacter sphaeroides hemA and hemT genes, encoding two 5-aminolevulinic acid synthase isozymes. Journal of Bacteriology, 175, 2292–2303.
Qin, G., Lin, J., Liu, X., & Cen, P. (2006). Effects of medium composition on production of 5-aminolevulinic acid by recombinant Escherichia coli. Journal of Bioscience and Bioengineering, 102, 316–322.
van der Werf, M. J., & Zeikus, J. G. (1996). 5-Aminolevulinate production by Escherichia coli containing the Rhodobacter sphaeroides hemA gene. Applied and Environmental Microbiology, 62, 3560–3566.
Xie, L., Hall, D., Eiteman, M. A., & Altman, E. (2003). Optimization of recombinant aminolevulinate synthase production in Escherichia coli using factorial design. Applied Microbiology and Biotechnology, 63, 267–273.
Chung, S. Y., Seo, K. H., & Rhee, J. I. (2005). Influence of culture conditions on the production of extra-cellular 5-aminolevulinic acid (ALA) by recombinant E. coli. Process Biochemistry, 40, 385–394.
Fu, W., Lin, J., & Cen, P. (2008). Enhancement of 5-aminolevulinate production with recombinant Escherichia coli using batch and fed-batch culture system. Bioresource Technology, 99, 4864–4870.
Andrews, S. C., Robinson, A. K., & Rodríguez-Quiñones, F. (2003). Bacterial iron homeostasis. FEMS Microbiology Reviews, 27, 215–237.
Kang, Z., Geng, Y., Xia, Y. z., Kang, J., & Qi, Q. (2009). Engineering Escherichia coli for an efficient aerobic fermentation platform. Journal of Biotechnology, 144, 58–63.
Yoon, S., Han, M.-J., Jeong, H., Lee, C., Xia, X.-X., Lee, D.-H., Shim, J., Lee, S., Oh, T., & Kim, J. (2012). Comparative multi-omics systems analysis of Escherichia coli strains B and K-12. Genome Biology, 13, R37.
Wang, X., Wang, Q., Qi, Q. (2015). Identification of riboflavin: revealing different metabolic characteristics between Escherichia coli BL21 (DE3) and MG1655. FEMS Microbiology Letters, 362, fnv071.
Han, M. J., Lee, S. Y., & Hong, S. H. (2012). Comparative analysis of envelope proteomes in Escherichia coli B and K-12 strains. Journal of Microbiology and Biotechnology, 22, 470–478.
Kim, B., Park, H., Na, D., & Lee, S. Y. (2014). Metabolic engineering of Escherichia coli for the production of phenol from glucose. Biotechnology Journal, 9, 621–629.
Qian, Z. G., Xia, X. X., Choi, J. H., & Lee, S. Y. (2008). Proteome-based identification of fusion partner for high-level extracellular production of recombinant proteins in Escherichia coli. Biotechnology and Bioengineering, 101, 587–601.
Li, N., Zhang, B., Chen, T., Wang, Z., Tang, Y.-J., & Zhao, X. (2013). Directed pathway evolution of the glyoxylate shunt in Escherichia coli for improved aerobic succinate production from glycerol. Journal of Industrial Microbiology & Biotechnology, 40, 1461–1475.
Xia, X.-X., Qian, Z.-G., Ki, C. S., Park, Y. H., Kaplan, D. L., & Lee, S. Y. (2010). Native-sized recombinant spider silk protein produced in metabolically engineered Escherichia coli results in a strong fiber. Proceedings of the National Academy of Sciences of the United States of America, 107, 14059–14063.
Tolia, N. H., & Joshua-Tor, L. (2006). Strategies for protein coexpression in Escherichia coli. Nature Methods, 3, 55–64.
Zhang, J., Kang, Z., Chen, J., & Du, G. (2015). Optimization of the heme biosynthesis pathway for the production of 5-aminolevulinic acid in Escherichia coli. Scientific Reports, 5, 8584.
Mauzerall, D., & Granick, S. (1956). The occurrence and determination of δ-aminolevulinic acid and porphobilinogen in urine. The Journal of Biological Chemistry, 219, 435–446.
Sassa, S. (1982). Delta-aminolevulinic acid dehydratase assay. Enzyme, 28, 133–145.
Frankenberg, N., Moser, J., & Jahn, D. (2003). Bacterial heme biosynthesis and its biotechnological application. Applied Microbiology and Biotechnology, 63, 115–127.
Franken, A. W., Lokman, B. C., Ram, A. J., Punt, P., Hondel, C. M. J. J., & Weert, S. (2011). Heme biosynthesis and its regulation: towards understanding and improvement of heme biosynthesis in filamentous fungi. Applied Microbiology and Biotechnology, 91, 447–460.
Schobert, M., & Jahn, D. (2002). Regulation of heme biosynthesis in non-phototrophic bacteria. Journal of Molecular Microbiology and Biotechnology, 4, 287–294.
Möbius, K., Arias-Cartin, R., Breckau, D., Hännig, A.-L., Riedmann, K., Biedendieck, R., Schröder, S., Becher, D., Magalon, A., Moser, J., Jahn, M., & Jahn, D. (2010). Heme biosynthesis is coupled to electron transport chains for energy generation. Proceedings of the National Academy of Sciences of the United States of America, 107, 10436–10441.
Touati, D. (2000). Iron and oxidative stress in bacteria. Archives of Biochemistry and Biophysics, 373, 1–6.
Massé, E., Salvail, H., Desnoyers, G., & Arguin, M. (2007). Small RNAs controlling iron metabolism. Current Opinion in Microbiology, 10, 140–145.
Kang, Z., Wang, X., Li, Y., Wang, Q., & Qi, Q. (2012). Small RNA RyhB as a potential tool used for metabolic engineering in Escherichia coli. Biotechnology Letters, 34, 527–531.
Li, F., Wang, Y., Gong, K., Wang, Q., Liang, Q., & Qi, Q. (2014). Constitutive expression of RyhB regulates the heme biosynthesis pathway and increases the 5-aminolevulinic acid accumulation in Escherichia coli. FEMS Microbiology Letters, 350, 209–215.
Panek, H., & O'Brian, M. R. (2002). A whole genome view of prokaryotic haem biosynthesis. Microbiology, 148, 2273–2282.
Lee, M. J., Kim, H. J., Lee, J. Y., Kwon, A. S., Jun, S. Y., Kang, S. H., & Kim, P. (2013). Effect of gene amplifications in porphyrin pathway on heme biosynthesis in a recombinant Escherichia coli. Journal of Microbiology and Biotechnology, 23, 668–673.
Acknowledgments
This work was financially supported by the National Natural Science Foundation of China (31200020), the Major State Basic Research Development Program of China (973 Program, 2013CB733602, 2014CB745103), the Jiangsu Planned Projects for Postdoctoral Research Funds (1301010B), the Program for Changjiang Scholars and Innovative Research Team in University (No. IRT1135), and the 111 Project.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Zhang, J., Kang, Z., Ding, W. et al. Integrated Optimization of the In Vivo Heme Biosynthesis Pathway and the In Vitro Iron Concentration for 5-Aminolevulinate Production. Appl Biochem Biotechnol 178, 1252–1262 (2016). https://doi.org/10.1007/s12010-015-1942-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-015-1942-2