Abstract
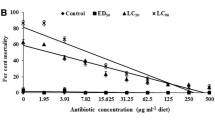
Helicoverpa armigera is one of the most important pests worldwide. Transgenic crops with toxin genes from Bacillus thuringiensis (Bt) have been deployed on a large scale to control this pest. The insecticidal activity of Bt is probably influenced by the insect midgut microbes, which vary across crop hosts and locations. Therefore, we examined the role of gut microbes in pathogenicity of Bt toxins in the H. armigera. Antibiotic cocktail was used for the complete elimination of the H. armigera gut microbes. Activated Cry1Ac, Bt formulation, and transgenic cotton resulted in larval weight loss and increase in mortality, but pretreatment of larvae with antibiotic cocktail significantly decreased larval mortality and increased the larval weight gain. Activated Cry1Ac and Bt formulation inhibited the activity of proteases in midgut of H. armigera larvae but showed no such effect in the larvae pretreated with antibiotic cocktail. Five protease bands in activated Cry1Ac and two in Bt formulation-treated larvae were inhibited but no such effect in the larvae pretreated with antibiotic cocktail. Cry1Ac protein was detected in Bt/Cry1Ac protoxin-fed larval gut extract in the absence of antibiotic cocktail, but fewer in larvae pretreated with antibiotic cocktail. The activity of antioxidant enzymes and aminopeptidases increased in larvae fed on Bt toxin, but there was no significant increase in antioxidant enzymes in larvae reared on toxin protein in combination with antibiotic cocktail. The results suggest that gut microbes exercise a significant influence on the toxicity of Cry1Ac and Bt formulation in H. armigera larvae. The implications of these results have been discussed in relation to development of insect resistance to Bt transgenic crops deployed for pest management.






Similar content being viewed by others
References
Sharma, H. C. (2005). Heliothis/Helicoverpa management: emerging trends and strategies for future research. New Delhi: Oxford and IBH Publishing Co.
Konecka, E., Kaznowski, A., Ziemnicka, J., & Ziemnicki, K. (2007). Molecular and phenotypic characterization of Bacillus thuringiensis isolated during epizootics in Cydia pomonella L. Journal of Invertebrate Pathology, 94, 56–63.
Brar, S., Verma, M., Tyagi, R., Surampalli, R., Bernabe, S., & Valero, J. (2007). Bacillus thuringiensis proteases: production and role in growth, sporulation and synergism. Process Biochemistry, 42, 773–790.
Bravo, A., Gomez, I., Conde, J., Garay, C. M., Sanchez, J., & Miranda, R. (2004). Oligomerization triggers binding of a Bacillus thuringiensis Cry1Ab pore-forming toxin to aminopeptidase N receptor leading to insertion into membrane micro domains. Biochimica et Biophysica Acta, 1667, 38–46.
Chen, D. Q., & Purcell, A. H. (1997). Occurrence and transmission of facultative endosymbionts in aphids. Current Microbiology, 34, 220–225.
Wilkinson, T. L. (1998). The elimination of intracellular microorganisms from insects: an analysis of antibiotic-treatment in the pea aphid (Acyrthosiphon pisum). Comparative Biochemistry and Physiology Part A, 119, 871–881.
Indiragandhi, P., Anandha, R., Madhaiyan, M., & Sa, T. M. (2008). Characterization of plant growth-promoting traits of bacteria isolated from larval guts of diamondback moth Plutella xylostella (Lepidoptera: Plutellidae). Current Microbiology, 56, 327–333.
Pardo, S. S., & Almeida, R. P. P. (2009). Role of symbiotic gut bacteria in the development of Acrosternum hilare and Murgantia histrionica (Hemiptera: Pentatomidae). Entomological Experimental et Applica, 132, 21–29.
Visotto, L. E., Oliveira, M. G. A., Guedes, R. N. C., Ribon, A. O. B., & Good-God, P. I. V. (2009). Contribution of gut bacteria to digestion and development of the velvetbean caterpillar, Anticarsia gemmatalis. Journal of Insect Physiology, 55, 185–191.
Paramasiva, I., Souche, Y., Kulakarni, G. J., Akbar, S. M. D., Sharma, H. C., & Krishnayya, P. V. (2014). Diversity in gut microflora of Helicoverpa armigera populations from different regions in relation to biological activity of Bacillus thuringiensis δ-endotoxin Cry1Ac. Archives of Insect Biochemistry and Physiology, 87, 201–213.
Broderick, N. A., Raffa, K. F., & Handelsman, J. (2006). Midgut bacteria required for Bacillus thuringiensis insecticidal activity. Proceedings of the National Academy of Sciences of the United States of America, 103, 15196–15199.
Broderick, N. A., Robinson, C. J., McMahon, M. D., Holt, J., Handelsman, J., & Raffa, K. F. (2009). Contributions of gut bacteria to Bacillus thuringiensis-induced mortality vary across a range of Lepidoptera. BMC Biology, 7, 11.
Johnston, P. R., & Crickmore, N. (2009). Gut bacteria are not required for the insecticidal activity of Bacillus thuringiensis toward the Tobacco Horn worm, Manduca sexta. Applied and Environmental Microbiology, 75, 5094–5099.
Raymond, B., Johnston, P. R., Wright, D. J., Ellis, R. J., Crickmore, N., & Bonsall, M. B. (2009). A midgut microbiota is not required for the pathogenicity of Bacillus thuringiensis to diamondback moth larvae. Environmental Microbiology, 11, 2556–2563.
Babu, G. C., Sharma, H. C., Madhumati, T., Raghavaiah, G., Murthy, K. V. M. K., & Rao, V. S. (2014). A semi-synthetic chickpea flour based diet for long-term maintenance of laboratory culture of Helicoverpa armigera. Indian Journal of Entomology, 76, 336–340.
Lowry, O. H., Rosebrough, N. J., Farr, A. L., & Randall, R. J. (1951). Protein measurement with the folin phenol reagent. Journal of Biological Chemistry, 193, 265–275.
Maa, X., Liu, X., Ning, X., Zhang, B., Han, F., Guan, X., Tan, Y., & Zhang, Q. (2008). Effects of Bacillus thuringiensis toxin Cry1Ac and Beauveria bassiana on Asiatic corn borer (Lepidoptera: Crambidae). Journal of Invertebrate Pathology, 99, 123–128.
Laemmli, U. K. (1970). Cleavage of structural proteins during assembly of the head of bacteriophage T4. Nature, 227, 680–685.
Vinod, D. P., Sharma, H. C., & Kochole, M. S. (2010). In vivo inhibition of Helicoverpa armigera gut pro-proteinase activation by non-host plant protease inhibitors. Journal of Insect Physiology, 56, 1315–1324.
Purushottam, R. L., & Vandana, K. H. (2013). Effect Bacillus thuringiensis (Bt) Cry1Ac toxin and protease inhibitor on growth and development of Helicoverpa armigera (Hubner). Pesticide Biochemistry and Physiology, 105, 77–83.
Li, H., Oppert, B., Higgins, R. A., Huang, F., Zhu, K. Y., & Buschman, L. L. (2004). Comparative analysis of proteinase activities of Bacillus thuringiensis-resistant and -susceptible Ostrinia nubilalis (Lepidoptera: Crambidae). Insect Biochemistry and Molecular Biology, 34, 753–762.
Garcia-Carreno, F. L., Dimes, L. E., & Haard, N. F. (1993). Substrate-gel electrophoresis for composition and molecular weight of proteinases or proteinaceous proteinase inhibitors. Analytical Biochemistry, 214, 65–69.
Wang, Y., Oberley, L. W., & Murhammer, D. W. (2001). Evidence of oxidative stress following the viral infection of two Lepidopteran insect cell lines. Free Radical Biology and Medicine, 31, 1448–1455.
McCord, J. M., & Fridovich, I. (1969). Superoxide dismutase: an enzymic function for erythro-cuprein (hemocuprein). Journal of Biological Chemistry, 244, 6049–6055.
Habig, W. H., Pabst, M. J., & Jakoby, W. B. (1974). Glutathione-S-transferases. Journal of Biological Chemistry, 249, 7130–7139.
Janero, D. R. (1990). Malondialdehyde and thiobarbituric acid-reactivity as diagnostic indices of lipid peroxidation and peroxidative tissue injury. Free Radical Biology and Medicine, 9, 515–540.
Wolfersberger, M. G., Luethy, P., Maurer, A., Parenti, P., Sacchi, F. V., Giordana, B., & Hanozet, G. M. (1987). Preparation and partial characterization of amino acid transporting brush bordered membrane vesicles from larval midgut of the cabbage butterfly (Pieris brassicae). Comparative Biochemistry and Physiology, 86, 301–308.
Nair, R., Kalia, V., Aggarwal, K. K., & Gujar, G. T. (2013). Variation in the cadherin gene sequence of Cry1Ac susceptible and resistant Helicoverpa armigera (Lepidoptera: Noctuidae) and the identification of mutant alleles in resistant strains. Current Science, 104, 215–223.
Wang, P., Zhao, J. Z., Simon, A. R., Kain, W., Janmaat, A. F., Shelton, A. M., Ferre, J., & Myers, J. (2007). Mechanism of resistance to Bacillus thuringiensis toxin Cry1Ac in a greenhouse population of the cabbage looper, Trichoplusia ni. Applied and Environmental Microbiology, 73, 1199–1207.
Keller, M., Sneh, B., Strizhov, N., Prudovsky, E., Regev, A., Koncz, C., Schell, J., & Zilberstein, A. (1996). Digestion of δ-endotoxin by gut proteases may explain reduced sensitivity of advanced instar larvae of Spodoptera littoralis to Cry1C. Insect Biochemistry and Molecular Biology, 26, 365–373.
Ogiwara, K., Indrasith, L. S., Asano, S., & Hori, H. (1992). Processing of δ-endotoxin from Bacillus thuringiensis subsp. Kurstaki HD-1 and HD-73 by gut juices of various insect larvae. Journal of Invertebrate Pathology, 60, 121–126.
Rajagopal, R., Arora, N., Sivakumar, S., Rao, N. G. V., Nimbalkar, S. A., & Bhatnagar, R. K. (2009). Resistance of Helicoverpa armigera to Cry1Ac toxin from Bacillus thuringiensis is due to improper processing of the protoxin. Biochemical Journal, 419, 309–316.
Oppert, B., Kramer, K. J., Johnson, D., Upton, S. J., & McGaughey, W. H. (1996). Proteinase-mediated insect resistance to Bacillus thuringiensis toxin. Insect Biochemistry and Molecular Biology, 26, 571–583.
Gunning, R. V., Dang, H. T., Kemp, F. C., Nicholson, I. C., & Graham, D. M. (2005). New resistance mechanism in Helicoverpa armigera threatens transgenic crops expressing Bacillus thuringiensis Cry1Ac toxin. Applied and Environmental Microbiology, 71, 2558–2563.
Frankenhuyzen, K. V., Liu, Y., & Amanda Tonon, A. (2010). Interactions between Bacillus thuringiensis subsp. kurstaki HD-1 and midgut bacteria in larvae of gypsy moth and spruce budworm. Journal of Invertebrate Pathology, 103, 124–131.
Paramasiva, I., Sharma, H. C., & Krishnayya, P. V. (2014). Antibiotics influence the toxicity of the delta endotoxins of Bacillus thuringiensis towards the cotton bollworm, Helicoverpa armigera. BMC Microbiology, 14, 200.
Hoeven, R. V., Betrabet, G., & Forst, S. (2008). Characterization of the gut bacterial community in Manduca sexta and effect of antibiotics on bacterial diversity and nematode reproduction. FEMS Microbiology Letters, 286, 249–256.
Somerville, H. J., Tanada, Y., & Omi, E. M. (1970). Lethal effect of purified spore and crystalline endotoxin preparations of Bacillus thuringiensis on several lepidopterous insects. Journal of Invertebrate Pathology, 16, 241–248.
Mohan, M., & Gujar, G. T. (2003). Characterization and comparison of midgut proteases of Bacillus thuringiensis susceptible and resistant diamondback moth (Plutellidae: Lepidoptera). Journal of Invertebrate Pathology, 82, 1–11.
Siqueira, H. A. A., Nickerson, K. W., Moellenbeck, D., & Siegfried, B. D. (2004). Activity of gut proteinases from Cry1Ab-selected colonies of the European corn borer, Ostrinia nubilalis (Lepidoptera: Crambidae). Pest Management Science, 60, 1189–1196.
Dubovskii, I. M., Olifirenko, O. A., & Glupov, V. V. (2005). Level and activities of antioxidants in intestine of larvae Galleria mellonella L. (Lepidoptera, Pyralidae) at peroral infestation by bacteria Bacillus thuringiensis ssp. galleriae. Journal of Evolutionary Biochemistry and Physiology, 41, 20–25.
Dubovskiy, I. M., Martemyanov, V. V., Vorontsova, Y. L., Rantala, M. J., Gryzanova, E. V., & Glupov, V. V. (2008). Effect of bacterial infection on antioxidant activity and lipid peroxidation in the midgut of Galleria mellonella L. larvae (Lepidoptera: Pyralidae). Comparative Biochemistry and Physiology - Part C, 148, 1–5.
Guo, J. Y., Wu, G., & Wan, F. H. (2010). Activities of digestive and detoxification enzymes in multiple generations of beet armyworm, Spodoptera exigua (Hubner), in response to transgenic Bt cotton. Journal of Pest Science, 83, 453–460.
Liao, C. Y., Trowell, S. C., & Akhuist, R. (2005). Purification and characterization of Cry1Ac toxin binding proteins from the brush border membrane of Helicoverpa armigera midgut. Current Microbiology, 51, 367–371.
Ingle, S. S., Trivedi, N., Prasad, R., Kuruvilla, J., Rao, K. K., & Chhatpar, H. S. (2001). Aminopeptidase-N from the Helicoverpa armigera (Hubner) brush border membrane vesicles as a receptor of Bacillus thuringiensis Cry1Ac δ-endotoxin. Current Microbiology, 43, 255–259.
Mason, K. L., Stepien, T. A., Blum, J. E., Labbe, N. H., Rush, J. S., Raffa, K. F., & Handelsman, J. (2011). From commensal to pathogen: translocation of Enterococcus faecalis from the midgut to the hemocoel of Manduca sexta. MBio, 2, 1–7.
Acknowledgments
We thank the staff, Insect Rearing Laboratory, Entomology, for providing the insects throughout the study.
Funding
This work was supported by UGC under UGC-SAP (DSR-I) and UGC-MRP scheme.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Visweshwar, R., Sharma, H.C., Akbar, S.M.D. et al. Elimination of Gut Microbes with Antibiotics Confers Resistance to Bacillus thuringiensis Toxin Proteins in Helicoverpa armigera (Hubner). Appl Biochem Biotechnol 177, 1621–1637 (2015). https://doi.org/10.1007/s12010-015-1841-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-015-1841-6