Abstract
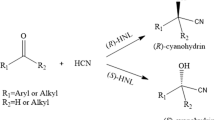
The carrier-based and carrier-free (cross-linked enzyme aggregate) covalent immobilizations of Prunus dulcis hydroxynitrile lyase were investigated. The immobilized preparations were tested for enantioselective carbon–carbon bond formation activity in the biphasic medium. Of the tested preparations, only cross-linked enzyme aggregate of P. dulcis hydroxynitrile lyase (PdHNL-CLEA) achieved the synthesis of (R)-mandelonitrile with 93 % yield and 99 % enantiopurity. PdHNL-CLEA was also used in the synthesis of various (R)-cyanohydrins from corresponding aldehydes/ketones and hydrocyanic acid. When 4-methoxybenzaldehyde, 4-methyl benzaldehyde, and 4-hydroxybenzaldehyde were used as substrates, the yield-enantiomeric excess of corresponding (R)-cyanohydrins were obtained as 95–95, 85–79, and 2–25 %, respectively, after 96 h at pH 4.0 and 5 °C. For acetophenone, 4-fluoroacetophenone, 4-chloroacetophenone, 4-bromoacetophenone, and 4-iodoacetophenone, the yield-enantiomeric excess of corresponding (R)-cyanohydrins were 1–99, 20–84, 11–95, 5–99, and 3–24 %, respectively at the same conditions. The results demonstrate PdHNL-CLEA can be effectively used in the synthesis of (R)-mandelonitrile.








Similar content being viewed by others
References
Lanfranchi, E., Steiner, K., Glieder, A., Hajnal, I., Sheldon, R. A., van Pelt, S., & Winkler, M. (2013). Mini-review: recent developments in hydroxynitrile lyases for industrial biotechnology. Recent Patents on Biotechnology, 7, 197–206.
Alagöz, D., Tükel, S. S., & Yildirim, D. (2014). Purification, immobilization and characterization of (R)-hydroxynitrile lyase from Prunus amygdalus turcomanica seeds and their applicability for synthesis of enantiopure cyanohydrins. Journal of Molecular Catalysis B: Enzymatic, 101, 40–46.
Winkler, M., Glieder, A., & Steiner, K. (2012). Comprehensive chirality. In E. M. Carreira & H. Yamamoto (Eds.), C-X bond formation: hydroxynitrile lyases: from nature to application (Vol. 7, pp. 350–371). Amsterdam: Elsevier B.V.
Torrelo, G., van Midden, N., Stloukal, R., & Hanefeld, U. (2014). Immobilized hydroxynitrile lyase: a comparative study of recyclability. ChemCatChem, 6, 1096–1102.
Hanefeld, U. (2013). Immobilisation of hydroxynitrile lyases. Chemical Society Reviews, 42, 6308–6321.
Wehtje, E., Adlercreutz, P., & Mattiasson, B. (1988). Activity and operational stability of immobilized mandelonitrile lyase in methanol/water mixtures. Applied Microbiology and Biotechnology, 29, 419–425.
Tükel, S. S., Yildirim, D., Alagöz, D., Alptekin, O., Yücebilgic, G., & Bilgin, R. (2010). Partial purification and immobilization of a new (R)-hydroxynitrile lyase from seeds of Prunus pseudoarmeniaca. Journal of Molecular Catalysis B: Enzymatic, 66, 161–165.
Cui, J.-D., Zhang, S., & Sun, L.-M. (2012). Cross-linked enzyme aggregates of phenylalanine ammonia lyase: novel biocatalysts for synthesis of l-phenylalanine. Applied Biochemistry and Biotechnology, 167, 835–844.
Illanes, A., Wilson, L., Caballero, E., Fernández-Lafuente, R., & Guisán, J. (2006). Crosslinked penicillin acylase aggregates for synthesis of β-lactam antibiotics in organic medium. Applied Biochemistry and Biotechnology, 133, 189–202.
Barbosa, O., Torres, R., Ortiz, C., & Fernandez-Lafuente, R. (2012). Versatility of glutaraldehyde to immobilize lipases: effect of the immobilization protocol on the properties of lipase B from Candida antarctica. Process Biochemistry, 47, 1220–1227.
Betancor, L., López-Gallego, F., Hidalgo, A., Alonso-Morales, N., Mateo, C., Fernández-Lafuente, R., & Guisán, J. M. (2006). Different mechanisms of protein immobilization on glutaraldehyde activated supports: effect of support activation and immobilization conditions. Enzyme and Microbial Technology, 39, 877–882.
Chmura, A., van Der Kraan, G. M., Kielar, F., van Langen, L. M., van Rantwijk, F., & Sheldon, R. A. (2006). Cross-linked aggregates of the hydroxynitrile lyase from Manihot esculenta: highly active and robust biocatalysts. Advanced Synthesis and Catalysis, 348, 1655–1661.
Yildirim, D., Tükel, S. S., & Alagöz, D. (2014). Crosslinked enzyme aggregates of hydroxynitrile lyase partially purified from Prunus dulcis seeds and its application for the synthesis of enantiopure cyanohydrins. Biotechnology Progress, 30, 818–827.
Yildirim, D., & Tükel, S. S. (2013). Immobilized Pseudomonas sp. lipase: a powerful biocatalyst for asymmetric acylation of (±)-2-amino-1-phenylethanols with vinyl acetate. Process Biochemistry, 48, 819–830.
Gunda, N. S. K., Singh, M., Norman, L., Kaur, K., & Mitra, S. K. (2014). Optimization and characterization of biomolecule immobilization on silicon substrates using (3-aminopropyl)triethoxysilane (APTES) and glutaraldehyde linker. Applied Surface Science, 305, 522–530.
Ansari, S. A., Satar, R., Chibber, S., & Khan, M. J. (2013). Enhanced stability of Kluyveromyces lactis β galactosidase immobilized on glutaraldehyde modified multiwalled carbon nanotubes. Journal of Molecular Catalysis B: Enzymatic, 97, 258–263.
Garcia-Galan, C., dos Santos, J. C. S., Barbosa, O., Torres, R., Pereira, E. B., Corberan, V. C., Gonçalves, L. R. B., & Fernandez-Lafuente, R. (2014). Tuning of Lecitase features via solid-phase chemical modification: effect of the immobilization protocol. Process Biochemistry, 49, 604–616.
Lowry, O. H., Rosebrough, N. J., Farr, A. L., & Randall, R. J. (1951). Protein measurement with the Folin phenol reagent. Journal of Biological Chemistry, 193, 265–275.
Alptekin, Ö., Tükel, S. S., Yildirim, D., & Alagöz, D. (2011). Covalent immobilization of catalase onto spacer-arm attached modified florisil: characterization and application to batch and plug-flow type reactor systems. Enzyme and Microbial Technology, 49, 547–554.
Hu, T.-G., Cheng, J.-H., Zhang, B.-B., Lou, W.-Y., & Zong, M.-H. (2015). Immobilization of alkaline protease on amino-functionalized magnetic nanoparticles and its efficient use for preparation of oat polypeptides. Industrial & Engineering Chemistry Research, 54, 4689–4698.
Yildirim, D., Tükel, S. S., Alagöz, D., & Alptekin, Ö. (2011). Preparative-scale kinetic resolution of racemic styrene oxide by immobilized epoxide hydrolase. Enzyme and Microbial Technology, 49, 555–559.
Laemmli, U. K. (1970). Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature, 227, 680–685.
Blum, H., Beier, H., & Gross, H. J. (1987). Improved silver staining of plant proteins, RNA and DNA in polyacrylamide gels. Electrophoresis, 8, 93–99.
van Langen, L. M., van Rantwijk, F., & Sheldon, R. A. (2003). Enzymatic hydrocyanation of a sterically hindered aldehyde. Optimization of a chemoenzymatic procedure for (R)-2-chloromandelic acid. Organic Process Research & Development, 7, 828–831.
Ueatrongchit, T., Tamura, K., Ohmiya, T., H-Kittikun, A., & Asano, Y. (2010). Hydroxynitrile lyase from Passiflora edulis: purification, characteristics and application in asymmetric synthesis of (R)-mandelonitrile. Enzyme and Microbial Technology, 46, 456–465.
Willeman, W. F., Hanefeld, U., Straathof, A. J. J., & Heijnen, J. J. (2000). Estimation of kinetic parameters by progress curve analysis for the synthesis of (R)-mandelonitrile by Prunus amygdalus hydroxynitrile lyase. Enzyme and Microbial Technology, 27, 423–433.
Nanda, S., Kato, Y., & Asano, Y. (2005). A new (R)-hydroxynitrile lyase from Prunus mume: asymmetric synthesis of cyanohydrins. Tetrahedron, 61, 10908–10916.
Roberge, C., Fleitz, F., Pollard, D., & Devine, P. (2007). Asymmetric synthesis of cyanohydrin derived from pyridine aldehyde with cross-linked aggregates of hydroxynitrile lyases. Tetrahedron Letters, 48, 1473–1477.
Acknowledgments
This study was financially supported by Cukurova University, Scientific Research Projects with the project number of IMYO2013BAP2.
Author information
Authors and Affiliations
Corresponding author
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
ESM 1
(DOCX 279 kb)
Rights and permissions
About this article
Cite this article
Alagöz, D., Tükel, S.S. & Yildirim, D. Enantioselective Synthesis of Various Cyanohydrins Using Covalently Immobilized Preparations of Hydroxynitrile Lyase from Prunus dulcis . Appl Biochem Biotechnol 177, 1348–1363 (2015). https://doi.org/10.1007/s12010-015-1819-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-015-1819-4