Abstract
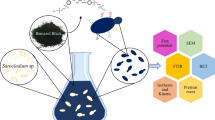
Adsorption is an efficient way to remove synthetic dyes from industrial effluent. Here, we show mechanism of adsorptive removal of cationic dye methylene blue (MB) from its aqueous solution using dried biomass of Rhizopus oryzae as a biosorbent. The optimum pH and temperature for adsorption was found to be 7.0 and 28 °C, respectively. Scanning electron microscopy (SEM) of the biomass suggested distinct changes in surface topology post-MB adsorption, while Fourier transform infrared (FTIR) study indicated chemical interaction between the surface of the biomass and MB. Chemical modification of –OH and –C=O groups of biomass reduced the MB adsorption and corroborated with the FTIR analyses. Kinetics study revealed that the adsorption rate was fast initially and reached equilibrium at 4 h following a pseudo-second-order-kinetics. The adsorption isotherm followed Freundlich isotherm model with n value of 1.1615.The dried biomass of R. oryzae can be used as a potent biosorbent for the removal of MB.







Similar content being viewed by others
References
Wanyonyi, W. C., Onyari, J. M., & Shiundu, P. M. (2013). Adsorption of methylene blue dye from aqueous solutions using Eichhornia crassipes. Bulletin of Environmental Contamination and Toxicology, 91, 362–366.
Hameed, B. H., et al. (2008). Equilibrium modeling and kinetic studies on the adsorption of basic dye by a low-cost adsorbent: coconut (Cocos nucifera) bunch waste. Journal of Hazardous Materials, 158, 65–72.
Hameed, B. H., Din, A. T. M., & Ahmad, A. L. (2007a). Adsorption of methylene blue onto bamboo-based activated carbon: kinetics and equilibrium studies. Journal of Hazardous Materials, 141, 819–825.
Hameed, B. H., Ahmad, A. L., & Latiff, K. N. A. (2007b). Adsorption of basic dye (methylene blue) onto activated carbon prepared from rattan saw dust. Dyes and Pigments, 75, 143–149.
Kavitha, D., & Namasivayam, C. (2007). Experimental and kinetic studies on methylene blue adsorption by coir pith carbon. Bioresource Technology, 98, 14–21.
Tan, I. A. W., Hameed, B. H., & Ahmad, A. L. (2007). Equilibrium and kinetic studies on basic dye adsorption by oil palm fibre activated carbon. Chemical Engineering Journal, 127, 111–119.
Tan, I. A. W., Ahmad, A. L., & Hameed, B. H. (2008). Optimization of preparation conditions for activated carbons from coconut husk using response surface methodology. Chemical Engineering Journal, 137, 462–470.
Das, S. K., Bhowal, J., Das, A. R., & Guha, A. K. (2006). Adsorption behaviour of rhodamine B on Rhizopus oryzae biomass. Langmuir, 22, 7265–7272.
Ahmad, A. A., Hameed, B. H., & Aziz, N. (2007). Adsorption of direct dye on palm ash: kinetic and equilibrium modeling. Journal of Hazardous Materials, 141, 70–76.
Hasan, M., Ahmad, A. L., & Hameed, B. H. (2008). Adsorption of reactive dye onto cross-linked chitosan/oil palm ash composite beads. Chemical Engineering Journal, 136, 164–172.
Batzias, F. A., & Sidiras, D. K. (2007). Simulation of methylene blue adsorption by salts-treated beech saw dust in batch and fixed bed systems. Journal of Hazardous Materials, 149, 8–17.
Bulut, Y., ¨oz¨ Ubenli, N. G., & Aydin, H. (2007). Equilibrium and kinetics for adsorption of direct blue 71 from aqueous solution by wheat shells. Journal of Hazardous Materials, 144, 300–306.
Singh, D. K., & Srivastava, B. (2001). Basic dyes removal from wastewater by adsorption on rice husk carbon. Indian Journal of Chemical Technology, 8, 133–139.
Annadurai, G., et al. (2002). Use of cellulose-based wastes for adsorption of dyes from aqueous solutions. Journal of Hazardous Materials, 92, 263–274.
Namasivayam, C., Kanchana, N., & Yamuna, R. T. (1993). Waste banana pith as adsorbent for removal of Rhodamine B from aqueous solution. Waste Management, 13, 89–95.
Namasivayam, C., Muniaswamy, N., Gayatri, K., Rani, M., & Ranganathan, K. (1996). Removal of dyes from aqueous solution by cellulosic waste orange peel. Bioresource Technology, 57, 37–43.
Namasivayam, C., Radhika, R., & Suba, S. (2001). Uptake of dyes by a promising locally available agricultural solid waste: coir pith. Waste Management, 21, 381–387.
Aksu, Z., & Tezer, S. (2000). Equilibrium and kinetic modeling of biosorption of remazol black B by Rhizopus arrhizus in a batch system: effect of temperature. Process Biochemistry, 36, 431–439.
Chatterjee, S., Chatterjee, S., Chatterjee, B. P., Das, A. R., & Guha, A. K. (2005). Adsorption of model anionic dye, eosin Y, from aqueous solution by chitosan hydrobeads. Journal of Colloid and Interface Science, 288, 30–35.
Ju, Y. H., Chen, T. C., & Liu, J. C. (1997). A study on the biosorption of lindane. Colloids and Surfaces, B: Biointerfaces, 9, 187–196.
Mogollon, L., Rodriguez, R., Larrota, W., Ramirez, N., & Torres, R. (1998). Biosorption of nickel using filamentous fungi. Applied Biochemistry and Biotechnology, 70–72, 593–601.
Panda, G. C., Das, S. K., & Guha, A. K. (2008). Biosorption of cadmium and nickel by functionalized husk of Lathyrus sativus. Colloids and Surfaces, B: Biointerfaces, 62, 173–179.
Panda, G. C., Das, S. K., & Guha, A. K. (2009). Jute stick powder as a potential biomass for the removal of Congo red and rhodamine B from their aqueous solution. Journal of Hazardous Materials, 164, 374–379.
Ho, Y. S., & McKay, G. (1998). Sorption of dye from aqueous solution by peat. Chemical Engineering Journal, 70, 115–124.
Ho, Y. S., & McKay, G. (2000). The kinetics of sorption of divalent metal ions onto sphagnum moss peat. Water Research, 34, 735–742.
Crini, G. (2006). Non-conventional low-cost adsorbents for dye removal: a review. Bioprocess Technology, 97, 1061–1085.
Ferrero, F. (2007). Dye removal by low cost adsorbents: hazel nut shells in comparison with wood saw dust. Journal of Hazardous Materials, 142, 144–152.
Weber, W. J., Jr., & Morris, J. C. (1963). Kinetics of adsorption on carbon from solution. Journal of the Sanitary Engineering Division Proceedings of the American Society of Civil Engineers, 89, 31–59.
Eastoe, J., & Dalton, J. S. (2000). Dynamic surface tension and adsorption mechanisms of surfactants at the air water interface. Advances in Colloid and Interface Science, 85, 103–144.
Foo, K. Y., & Hameed, B. H. (2010). Insights into the modeling of adsorption isotherm systems. Chemical Engineering Journal, 156, 2–10.
Dawodu, F. A., Akpomie, G. K., & Ogbu, L. C. (2012). Isotherm modeling on the equilibrium sorption of cadmium (II) from solution by Agbani Clay. International Journal of Multidisciplinary Science And Engineering, 3(9), 9–14.
Acknowledgments
The authors acknowledge the research support provided by Tezpur University, Tezpur. RM acknowledges funds received from the Department of Biotechnology Govt. of India and start-up funds from Tezpur University. Authors sincerely thank Dr. L.G. Roy, Jadavpur University, Kolkata, India for the fungal strain.
Conflict of Interest
The authors declare that they have no competing interests.
Research Involving Human Participants and/or Animals
No
Informed Consent
Not applicable
Ethical Statements from Corresponding Author
• The manuscript has not been submitted to more than one journal for simultaneous consideration.
• The manuscript has not been published previously (partly or in full), unless the new work concerns an expansion of previous work (please provide transparency on the re-use of material to avoid the hint of text-recycling (“self-plagiarism”)).
• A single study is not split up into several parts to increase the quantity of submissions and submitted to various journals or to one journal over time (e.g., “salami-publishing”).
• No data have been fabricated or manipulated (including images) to support your conclusions.
• No data, text, or theories by others are presented as if they were the author’s own (“plagiarism”). Proper acknowledgements to other works must be given (this includes material that is closely copied (near verbatim), summarized and/or paraphrased); quotation marks are used for verbatim copying of material, and permissions are secured for material that is copyrighted.
• Consent to submit has been received explicitly from all co-authors, as well as from the responsible authorities—tacitly or explicitly—at the institute/organization where the work has been carried out, before the work is submitted.
• Authors whose names appear on the submission have contributed sufficiently to the scientific work and therefore share collective responsibility and accountability for the results.
Author information
Authors and Affiliations
Corresponding author
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Supplementary Fig. S1
(DOCX 252 kb)
Rights and permissions
About this article
Cite this article
Dey, M.D., Shukla, R., Bordoloi, N.K. et al. Mechanism of Adsorptive Removal of Methylene Blue Using Dried Biomass of Rhizopus oryzae . Appl Biochem Biotechnol 177, 541–555 (2015). https://doi.org/10.1007/s12010-015-1761-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-015-1761-5