Abstract
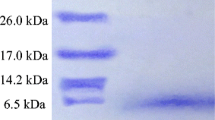
Bacterial infections causing fish diseases and spoilage during fish food processing and storage are major concerns in aquaculture. Use of bacteriocins has recently been considered as an effective strategy for prevention of bacterial infections. A novel bacteriocin produced by Catla catla gut isolates, Lactobacillus animalis TSU4, designated as bacteriocin TSU4 was purified to homogeneity by a three-step protocol. The molecular mass of bacteriocin TSU4 was 4117 Da determined by Q-TOF LC/MS analysis. Its isoelectric point was ~9. Secondary conformation obtained by circular dichroism spectroscopy showed molecular conformation with significant proportions of the structure in α-helix (23.7 %) and β-sheets (17.1 %). N-terminal sequencing was carried out by the Edman degradation method; partial sequence identified was NH2-SMSGFSKPHD. Bacteriocin TSU4 exhibited a wide range of antimicrobial activity, pH and thermal stability. It showed a bacteriocidal mode of action against the indicator strain Aeromonas hydrophila MTCC 646. Bacteriocin TSU4 is the first reported bacteriocin produced by fish isolate Lactobacillus animalis. The characterization of bacteriocin TSU4 suggested that it is a novel bacteriocin with potential value against infections of bacteria such as A. hydrophila MTCC 646 and Pseudomonas aeruginosa MTCC 1688 and application to prevent spoilage during food preservation.





Similar content being viewed by others
References
Hassan, M., Kjos, M., Nes, I. F., Diep, D. B., & Lotfipour, F. (2012). Natural antimicrobial peptides from bacteria: characteristics and potential applications to fight against antibiotic resistance. Journal of Applied Microbiology, 113, 723–736.
Singh, N., & Abraham, J. (2014). Ribosomally synthesized peptides from natural sources. The Journal of Antibiotics, 67, 277–289.
Bali, V., Panesar, P. S., Bera, M. B., & Kennedy, J. F. (2014). Bacteriocins: recent trends and potential applications. Critical Reviews in Food Science and Nutrition. doi:10.1080/10408398.2012.729231.
Chahad, O. B., Bour, M. E., Calo-Mata, P., Boudabous, A., & Barros-Velazquez, J. (2012). Discovery of novel biopreservation agents with inhibitory effects on growth of food-borne pathogens and their application to seafood products. Research in Microbiology, 163, 44–54.
Sugita, H., Matsuo, N., Hirose, Y., Iwato, M., & Deguchi, Y. (1997). Vibrio sp. strain NM 10, isolated from the intestine of a Japanese coastal fish, has an inhibitory effect against Pasteurella piscicida. Applied and Environmental Microbiology, 63, 4986–4989.
Balcazar, J. L., De Blas, I., Ruiz-Zarzuela, I., & Vendrell, D. (2007). Enhancement of the immune response and protection induced by probiotic lactic acid bacteria against furunculosis in rainbow trout (Oncorhynchus mykiss). FEMS Immunology and Medical Microbiology, 51, 185–193.
Ghosh, S., Ringo, E., Selvam, A. D. G., Rahiman, K. M. M., Sathyan, N., Nifty, J., et al. (2014). Gut associated lactic acid bacteria isolated from the estuarine fish Mugil cephalus: molecular diversity and antibacterial activities against pathogens. International Journal of Aquaculture, 4, 1–11. doi:10.5376/ija.2014.04.0001.
Calo-Mata, P., Arlindo, S., Boehme, K., & de Miguel, T. (2008). Current applications and future trends of lactic acid bacteria and their bacteriocins for the biopreservation of aquatic food products. Food and Bioprocess Technology, 1, 43–63.
Giri, S. S., Sukumaran, V., Sen, S. S., & Vinumonia, J. (2011). Antagonistic activity of cellular components of potential probiotic bacteria, isolated from the gut of Labeo rohita, against Aeromonas hydrophila. Probiotics and Antimicrobial Proteins, 3, 214–222.
Holck, A., Axelssons, L., Birkeland, S. E., Aukrust, T., & Blom, H. (1992). Purification and amino acid sequence of sakacin A, a bacteriocin from Lactobacillus sake Lb706. Journal of General Microbiology, 138, 2715–2720.
Iyapparaj, P., Maruthiah, T., Ramasubburayan, R., Prakash, S., Kumar, C., Immanuel, G., et al. (2013). Optimization of bacteriocin production by Lactobacillus sp. MSU3IR against shrimp bacterial pathogens. Aquatic Biosystems. doi:10.1186/2046-9063-9-12.
Amortegui, J., Rodríguez-López, A., Rodríguez, D., Carrascal, A. K., Alméciga-Díaz, C. J., Melendez, A. D. P., et al. (2014). Characterization of a new bacteriocin from Lactobacillus plantarum LE5 and LE27 isolated from ensiled corn. Applied Biochemistry and Biotechnology. doi:10.1007/s12010-014-0757-x.
Sahoo, T. K., Jena, P. K., Patel, A. K., & Seshadri, S. (2014). Bacteriocins and their applications for the treatment of bacterial diseases in aquaculture: a review. Aquaculture Research. doi:10.1111/are.12556.
Messi, P., Guerrieri, E., & Bondi, M. (2003). Bacteriocin-like substance (BLS) production in Aeromonas hydrophila water isolates. FEMS Microbiology Letters, 220, 121–125.
Sahoo, T. K., Jena, P. K., Nagar, N., Patel, A. K., & Seshadri, S. (2015). In vitro evaluation of probiotic properties of lactic acid bacteria from the gut of Labeo rohita and Catla catla. Probiotics and Antimicrobial Proteins. doi:10.1007/s12602-015-9184-8.
Stoffels, G., Nissen-Meyer, J., Gudmundsdottir, A., & Sletten, K. (1992). Purification and characterization of a new bacteriocin isolated from a Carnobacterium sp. Journal of Bacteriology, 73, 309–316.
Bradford, M. M. (1976). A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Analytical Biochemistry, 72, 248–254.
Uteng, M., Hauge, H. H., Brondz, I., Nissen-Meyer, J., & Fimland, G. (2002). Purification and characterization of a novel class IIa bacteriocin, piscicocin CS526, from surimi-associated Carnobacterium piscicola CS526. Applied and Environmental Microbiology, 68, 952–956.
Schagger, H., & Jagow, G. V. (1987). Tricine–sodium dodecyl sulphate–polyacrylamide gel electrophoresis for the separation of proteins in the range from 1 to 100 kDa. Analytical Biochemistry, 166, 368–379.
Dimitrijevic, R., Stojanovic, M., Ivkovic, I. Z., Petersen, A., Jankov, R. M., Dimitrijevic, L., et al. (2009). The identification of a low molecular mass bacteriocin, rhamnosin A, produced by Lactobacillus rhamnosus strain 68. Journal of Applied Microbiology, 107, 2108–2115.
Tiwari, S. K., & Srivastava, S. (2008). Purification and characterization of plantaricin LR14: a novel bacteriocin produced by Lactobacillus plantarum LR/14. Applied Microbiology and Biotechnology, 79, 759–767.
Netz, D. J. A., Pohl, R., Beck-Sickinger, A. G., Selmer, T., Pierik, A. J., Bastos, M. D. C. D. F., et al. (2002). Biochemical characterization and genetic analysis of aureocin A53, a new, atypical bacteriocin from Staphylococcus aureus. Journal of Molecular Biology, 319, 745–756.
Rogers, A. M., & Montville, T. J. (1991). Improved agar diffusion assay for nisin quantification. Food Biotechnology, 5, 161–168.
Jena, P. K., Trivedi, D., Chaudhary, H., Sahoo, T. K., & Seshadri, S. (2013). Bacteriocin PJ4 active against enteric pathogen produced by Lactobacillus helveticus PJ4 isolated from gut microflora of Wistar Rat (Rattus norvegicus): partial purification and characterization of bacteriocin. Applied Biochemistry and Biotechnology, 169, 2088–2100.
Ghanbari, M., Jami, M., Kneifel, W., & Domig, K. J. (2013). Antimicrobial activity and partial characterization of bacteriocins produced by lactobacilli isolated from Sturgeon fish. Food Control, 32, 379–385.
Yanagida, F., Chen, Y., & Shinohara, T. (2006). Searching for bacteriocin-producing lactic acid bacteria in soil. The Journal of General and Applied Microbiology, 52, 21–28.
Maldonado, A., Ruiz-Barba, J. L., & Jimenez-Diaz, R. (2003). Purification and genetic characterization of plantaricin NC8, a novel coculture-inducible two-peptide bacteriocin from Lactobacillus plantarum NC8. Applied and Environmental Microbiology, 69, 383–389.
Yamazaki, K., Suzuki, M., Kawai, Y., Inoue, N., & Montville, T. J. (2005). Purification and characterization of a novel class IIa bacteriocin, Piscicocin CS526, from surimi-associated Carnobacterium piscicola CS526. Applied and Environmental Microbiology, 71, 554–557.
Bendjeddou, K., Fons, M., Strocker, P., & Sadoun, D. (2012). Characterization and purification of a bacteriocin from Lactobacillus paracasei subsp. paracasei BMK2005, an intestinal isolate active against multidrug-resistant pathogens. World Journal of Microbiology and Biotechnology, 28, 1543–1552.
Ray, B., Miller, K. W., & Jain, M. K. (2001). Bacteriocins of lactic acid bacteria: current perspectives. Indian Journal of Microbiology, 41, 1–21.
Gonda, D. K., Bachmair, A., Wunnin, I., & Tobias, J. W. (1989). Universality and structure of the N-end rule. The Journal of Biological Chemistry, 264, 16700–16712.
Chen, Y., Wang, Y., Chow, Y., & Yanagida, F. (2014). Purification and characterization of plantaricin Y, a novel bacteriocin produced by Lactobacillus plantarum 510. Achieves of Microbiology, 196, 193–199.
Ennahar, S., Sashihara, T., Sonomoto, K., & Ishizaki, A. (2000). Class IIa bacteriocins: biosynthesis, structure and activity. FEMS Microbiology Reviews, 24, 85–106.
Cotter, P. D., Hill, C., & Ross, P. R. (2005). Bacteriocins: developing innate immunity for food. Nature Reviews Microbiology, 3, 777–788.
Breukink, E., & de Kruijff, B. (2006). Lipid II as a target for antibiotics. Nature Reviews Drug Discovery, 5, 321–323.
Hu, P., Dang, Y., Liu, B., & Lu, X. (2014). Purification and partial characterization of a novel bacteriocin produced by Lactobacillus casei TN-2 isolated from fermented camel milk (Shubat) of Xinjiang Uygur Autonomous region, China. Food Control. doi:10.1016/j.foodcont.2014.03.020.
Acknowledgments
The authors are thankful to Intas Pharmaceutical Limited (Biopharma Division), Nirma Education and Research Foundation (NERF), Ahmedabad (India) and School of Life Sciences, Sambalpur University, Sambalpur (India), for providing research and infrastructure facilities. The authors are also thankful to Mr. Rajender Jena, Ph.D. Scholar, Kusuma School of Biological Sciences, Indian Institute of Technology, Delhi (India), for his technical support.
Conflict of Interest
There is no conflict of interest among the authors.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Sahoo, T.K., Jena, P.K., Patel, A.K. et al. Purification and Molecular Characterization of the Novel Highly Potent Bacteriocin TSU4 Produced by Lactobacillus animalis TSU4. Appl Biochem Biotechnol 177, 90–104 (2015). https://doi.org/10.1007/s12010-015-1730-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-015-1730-z