Abstract
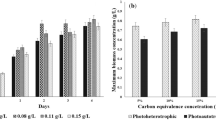
Following the diminishing hopes from the first and second generation biofuels, mainly due to the limitations of land availability, feed stock requirements, and complicated pre-treatments, third generation biofuels from microalgae are becoming a priority in the current scenario. The present study focuses on comparison and optimization of lipid accumulation efficiency in algal strain Chlorella minutissima grown under autotrophic, heterotrophic, and mixotrophic modes of nutrition, employing various carbon sources obtained from cheap industrial wastes such as glucose, acetate, and glycerol. Other pertinent factors such as the effect of various nitrogen sources, effect of salinity on the cell growth, and lipid accumulations in the algal cells were also studied. The results suggested that C. minutissima can grow efficiently under autotrophic, heterotrophic, and mixotrophic modes of nutrition. C. minutissima cells were capable of utilizing other non-popular carbon sources such as glycerol and acetate collected as waste products from different industries along with commonly used glucose. The maximum biomass concentration (8.9 g/L) and lipid content (36.19 %) were found in heterotrophic mode of nutrition. Our findings indicated that C. minutissima can efficiently utilize these cheaper carbon sources from industrial waste products for its growth and the production cost of various bioenergy sources can be reduced significantly.






Similar content being viewed by others
References
Heredia-Arroyo, T., Wei, W., Ruan, R., & Hu, B. (2011). Mixotrophic cultivation of Chlorella vulgaris and its potential application for the oil accumulation from non-sugar materials. 35, 2245-2253.
Oo, T.T., Weine Oo, N.N., Thu, M.K., & Oo, M.M. (2008). Optimization of oil-rich microalgae (Scenedesmus sp. and Chlorella sp.) culture for biodiesel research and development. The Third GMSARN International Conference. 35.
Boyle, G. (2012). Renewable energy: power for a sustainable future (3rd ed.). USA: Oxford University Press.
Peterson, C. L., & Hustrulid, T. (1998). Carbon cycle for rapeseed oil biodiesel fuels. Biomass and Bioenergy, 14, 91–101.
Carlsson, A.S., Van Beilen, J.B., Moller, R., Clayton, D. (2007). In: Bowles D, editor. Micro- and macro-algae: utility for industrial applications, outputs from the EPOBIO project. University of York. CPL Press, Newbury (UK).
Schenk, M. P., Thomas-Hall, S. R., Stephens, E., Marx, U. C., Mussgnug, J. H., Posten, C., Kruse, O., & Hankamer, B. (2008). Second generation biofuels: high-efficiency microalgae for biodiesel production. Bioenergy Resarch, 1, 20–43.
Aquafuels: algae towards biofuels (2010). Available from: http://www.aquafuels.eu/project-info.html [accessed 23.04.2015].
Miao, X., Wu, Q., & Yang, C. (2004). Fast pyrolysis of microalgae to produce renewable fuels. Journal of Applied Pyrolysis, 71, 855–863.
Chisti, Y. (2007). Biodiesel from microalgae. Biotechnology Advances, 25(3), 294–306.
Gouveia, L., (2011). Microalgae as a feedstock for biofuels. Springer Briefs in Microbiology. 1–68.
Darzins, A., Pienkos, P., & Edye, L. (2010). Current status and potential for algal biofuels production. Bioindustry partners and NERL. Bioenergy Task, 39, 131.
Bozbas, K. (2008). Biodiesel as an alternative motor fuel: production and policies in the European Union. Renewable and Sustainable Energy Reviews, 12, 542–552.
Miao, X., & Wu, Q. (2006). Biodiesel production from heterotrophic microalgal oil. Bioresource Technology, 97, 841–846.
Lee, Y.K. (2003). Algal nutrition: heterotrophic carbon nutrition. In: Richmond A, Ed., Biotechnology and applied phycology. Handbook of microalgal culture. Blackwell Publishing, 116.
Xu, H., Miao, X., & Wu, Q. (2006). High quality biodiesel production from a microalgae Chlorella protothecoides by heterotrophic growth in fermenters. Journal of Biotechnology, 126, 499–507.
Droop, M.R., (1974). Heterotrophy of carbon. In Stewart WDP, Ed., Algal physiology and biochemistry. University of California Press. 530–559.
Martinez, F., & Orus, M. I. (1991). Interactions between glucose and inorganic carbon metabolism in Chlorella vulgaris strain UAM101. Plant Physiology, 95, 1150–1155.
Liang, Y., Sarkany, N., & Cui, Y. (2009). Biomass and lipid productivities of Chlorella vulgaris under autotrophic, heterotrophic and mixotrophic grown conditions. Biotechnology Letters, 31, 1043–1049.
Xiong, W., Li, X. F., Xiang, J. Y., & Wu, Q. Y. (2008). High-density fermentation of microalgae Chlorella protothecoides in bioreactor for micro-biodiesel production. Applied Microbiology and Biotechnology, 78, 29–15.
Heredia-Arrayo, T., Wei, W., & Hu, B. (2010). Oil accumulation via heterotrophic/mixotrophic Chlorella protothecoides. Applied Biochemistry and Biotehnology, 162, 1978–1995.
Dubois, M., Gilles, K. A., Hamilton, J. K., Rebers, P. A., & Smith, F. (1956). Colorimetric method for determination of sugars and related substances. Analytical Chemistry, 28(3), 350–356.
Bligh, E. G., & Dyer, W. J. (1959). A rapid method of total lipid extraction and purification. Canadian Journal of Biochemistry and Physiology, 37, 911–917.
Kong, W., Yang, H., Cao, Y., Song, H., Hua, S., & Xia, C. (2013). Effect of glycerol and glucose on the enhancement of biomass, lipid and soluble carbohydrate production by Chlorella vulgaris in mixotrophic culture. Food Technology and Biotechnology, 51(1), 62–69.
Barclay, B. (1984). Microalgae culture collection. Microalgal Technology Research Group (MTRG). SERI/SP-231-2486.
Chiu, S. Y., Kao, C. Y., Tsai, M. T., Ong, S. C., Chen, C. H., & Lin, C. S. (2009). Lipid accumulation and CO2 utilization of Nannochloropsis oculata in response to CO2 aeration. Bioresource Technology, 100, 833–838.
Daniela, M. S., Raunel, T., John, K., & Alfredo, M. (2013). Heterotrophic growth of Neochloris oleoabundans using glucose as a carbon source. Biotechnology for Biofuels, 6(100), 1–12.
David, U. S., Xavier, F., Antoni, S. F., Raquel, B., Sergio, R., & Angel, V. (2015). Valorisation of biodiesel production wastes: anaerobic digestion of residual Tetraselmis suecica biomass and co-digestion with glycerol. Waste Management & Research, 33, 250–257.
Demirbas, A. (2009). Progress and recent trends in biodiesel fuels. Energy Conversion and Management, 50, 14–34.
DeMorais, M. G., & Costa, J. A. V. (2007). Carbon dioxide fixation by Chlorella kessleri, C. vulgaris, Scenedesmus obliquus and Spirulina sp. cultivated in flasks and vertical tubular photobioreactors. Biotechnology Letters, 29(9), 1349–1352.
Drora, K., Zvi, C., & Aharon, A. (1986). Optimal growth conditions for Isochrysis galbana. Biomass, 9, 37–48.
Eriksen, N. T. (2008). The technology of microalgal culturing. Biotechnology Letters, 30, 1525–1536.
Feinberg, D.A., (1984). Fuel options from microalgae with representative chemical compositions. SERI/TR-231-2427.
Gouveia, L., & Oliveira, A. C. (2009). Microalgae as a raw material for biofuels production. Journal of Industrial Microbiology and Biotechnology, 36, 269–274.
Grima, E. M., Fernandez, F. G. A., Camacho, F. G., Rubio, F. C., & Chisti, Y. (2000). Scale-up of tubular photobioreactors. Journal of Applied Phycology, 12, 355–68.
Hanhua, H. K. G. (2006). Response of growth and fatty acid compositions of Nannochloropsis sp. to environmental factors under elevated CO2 concentration. Biotechnology Letters, 28, 987–992.
Henderson, R. J., John, W. L., & John, R. S. (1988). Lipid composition and biosynthesis in the marine dinoflagellate Crypthecodinium cohnii. Phytochemistry, 27(6), 1679–1683.
Heredia-Arroyo, T., Wei, W., Ruan, R., & Hu, B. (2011). Mixotrophic cultivation of Chlorella vulgaris and its potential application for the oil accumulation from non-sugar materials. Biomass and Bioenergy, 35, 2245–2253.
Huntley, M. E., & Redalje, D. G. (2007). CO2 mitigation and renewable oil from photosynthetic microbes: a new appraisal. Mitigation and Adaptation Strategies for Global Change, 12(4), 573–608.
Illman, A. M., Scragg, A. H., & Shales, S. W. (2000). Increase in Chlorella strains calorific values when grown in low nitrogen medium. Enzyme and Microbial Technology, 27, 631–635.
Jianhua, F., Yanbin, C., Minxi, W., Weiliang, W., & Yuanguang, L. (2014). Lipid accumulation and biosynthesis genes response of the oleaginous Chlorella pyrenoidosa under three nutrition stressors. Biotechnology for Biofuels, 7, 1–14.
Jiao, X., Ying-Fang, N., Tan, H., Wei-Dong, Y., Jie-Sheng, L., & Hong-Ye, L. (2015). Genetic improvement of the microalga Phaeodactylum tricornutum for boosting neutral lipid accumulation. Metabolic Engineering, 27, 1–9.
Jun, C., Jia, F., Jing, S., Yun, H., Junhu, Z., & Kefa, C. (2014). Enhancing the lipid content of the diatom Nitzschia sp. by 60Co-γ irradiation mutation and high-salinity domestication. Energy, 78, 9–15.
Leathers, J., Celina, M., Chianelli, R., Thoma, S., Gupta, V., (2007). Systems analysis and futuristic designs of advanced biofuel factory, concepts. SANDIA Report, SAND, 6872.
Lee, Y. K. (2001). Microalgal mass culture systems and methods: their limitation and potential. Journal of Applied Phycology, 13, 307–315.
Li, Y., Horsman, M., Wu, N., Lan, C. Q., & Dubois-Calero, N. (2008). Biofuels from microalgae. Biotechnology Progress, 24(4), 815–20.
Mahapatra, D. M., & Ramachandra, T. V. (2013). Algal biofuel: bountiful lipid from Chlorococcum sp. proliferating in municipal wastewater. Current Science, 105(1), 47–55.
Mata, M. T., Martins, A. A., & Caetano, N. S. (2010). Microalgae for biodiesel production and other applications: a review. Renewable and Sustainable Energy Reviews, 14, 217–323.
Melinda, J. G., & Susan, T. L. H. (2009). Lipid productivity as a key characteristic for choosing algal species for biodiesel production. Journal of Applied Phycology, 21, 493–507.
Michiki, H. (1995). Biological CO2 fixation and utilization project. Energy Conversion and Management, 36(6–9), 701–705.
Minowa, R., Yokoyama, S., Kishimoto, M., & Okakurat, T. (1995). Oil production from algal cells of Dunaliella tertiolecta by direct thermochemical liquefaction. Fuel, 74(12), 1735–1738.
Mohamad, A. S. (2013). Heterotophic growth of Ankistrodesmus sp. for lipid production using cassava starch hydrolysate as a carbon source. The International Journal of Biotechnology, 2(1), 42–51.
Moheimani, N.R., (2005). The culture of Coccolithophorid algae for carbon dioxide bioremediation. PhD thesis. Murdoch University.
Moheimani, N.R., Borowitzka, M.A., (2006). The long-term culture of the coccolithophore Pleurochrysis carterae (Haptophyta) in outdoor raceway ponds. Journal of Applied Phycology, 18, 703–712.
Momocha, N., Sachitra, K. R., Radha, P., Anil, K. S., Dolly, W. D., Chandragiri, S., & Rachapudi, B. N. P. (2012). Biochemical modulation of growth, lipid quality and productivity in mixotrophic cultures of Chlorella sorokiniana. Springer Plus, 1, 1–33.
Nagaraja, Y. P., Biradar, C., Manasa, K. S., & Venkatesh, H. S. (2014). Production of biofuel by using micro algae (Botryococcus braunii). International Journal of Current Microbiology and Applied Sciences, 3(4), 851–860.
Natrah, F., Yoso, V. F. M., Shari, V. M., Abas, F., & Mariana, N. S. (2007). Screening of Malaysian indigenous microalgae for antioxidant properties and nutritional value. Journal of Applied Phycology, 19, 711–718.
Negoro, M., Shioji, N., Miyamoto, K., & Miura, Y. (1991). Growth of microalgae in high CO2 gas and effects of SOX and NOX. Applied Biochemistry and Biotechnology—Part A Enzyme Engineering and Biotechnology, 28–29, 877–886.
Peng, W., Wu, Q., Tu, P., & Zhao, N. (2001). Pyrolitic characteristics of microalgae as renewable energy source determined by thermogravimetric analysis. Bioresource Technology, 80, 1–7.
Poisson, L., Devos, M., Pencreach, G., & Ergan, F. (2002). Benefits and current developments of polyunsaturated fatty acids from microalgae lipids. OCL—Oleagineux Corps Gras Lipides, 9(2-3), 92–95.
Rajiv, C. D. G., & Kalita, M. C. (2011). Scenedesmus dimorphus and Scenedesmus quadricauda: two potent indigenous microalgae strains for biomass production and CO2 mitigation—a study on their growth behavior and lipid productivity under different concentration of urea as nitrogen source. Journal of Algal Biomass Utilization, 2, 42–49.
Raymond, K., Indah, R., & Muhammad, Z. (2014). Production of biodiesel from lipid of phytoplankton Chaetoceros calcitrans through ultrasonic method. The Scientific World Journal, 9, 231361.
Renaud, S. M., Thinh, L. V., & Parry, D. L. (1997). The gross chemical composition and fatty acid composition of 18 species of tropical Australian microalgae for possible use in mariculture. Aquaculture, 170, 147–59.
Richmond, A., (2004). Handbook of microalgal culture: biotechnology and applied. Phycology. Blackwell Science Ltd.
Rodolfi, L., Zittelli, G. C., Bassi, N., Padovani, G., Biondi, N., Bonini, G., & Tredici, M. R. (2009). Microalgae for oil: strain selection, induction of lipid synthesis and outdoor mass cultivation in a low-cost photobioreactor. Biotechnology and Bioengineering, 102(1), 100–12.
Saddam, H. A., Talal, Y., & Raed, A. A. (2014). Biofuels from the fresh water microalgae Chlorella vulgaris (FWM-CV) for diesel engines. Energies, 7, 1829–1851.
Sancho, M. E. M., Castillo, J. M. J., & El, Y. F. (1999). Photoautotrophic consumption of phosphorus by Scenedesmus obliquus in a continuous culture. Influence of light intensity. Process Biochemistry, 34(8), 811–818.
Sawayama, S., Minowa, T., & Yokoyama, S. Y. (1999). Possibility of renewable energy production and CO2 mitigation by thermochemical liquefaction of microalgae. Biomass and Bioenergy, 17(1), 33–39.
Scragg, A. H., Illman, A. M., Carden, A., & Shales, S. W. (2002). Growth of microalgae with increased calorific values in a tubular bioreactor. Biomass and Bioenergy, 23(1), 67–73.
Singh, S. K., Bansal, A., Jha, M. K., & Jain, R. (2013). Production of biodiesel from wastewater grown Chlorella minutissima. Indian Journal of Chemical Technology, 20, 341–345.
Spolaore, P., Joannis-Cassan, C., Duran, E., & Isambert, A. (2006). Commercial applications of microalgae. Journal of Bioscience and Bioengineering, 101(2), 87–96.
Thomas, W. H., Seibert, D. L. R., Alden, M., Eldridge, P., & Neori, A. (1983). Yields, photosynthetic efficiencies and proximate composition of dense marine microalgal cultures II. Dunaliella primolecta and Tetraselmis suecica experiments. Biomass, 5, 211–225.
Thomas, W.H., Tornabene, T.G., Weissman, J. (1984). Screening for lipid yielding microalgae: activities for 1983. SERI/STR-231-2207.
Tri, P., Mujizat, K., & Vicky, K. (2013). Fatty acid composition of three diatom species Skeletonema costatum, Thalassiosira sp. and Chaetoceros gracilis. International Journal of Environment and Bioenergy, 6(1), 28–43.
Ugwu, C. U., Aoyagi, H., & Uchiyama, H. (2008). Photobioreactors for mass cultivation of algae. Bioresource Technology, 99(10), 4021–4028.
Wu, Y. H., Yang, J., Hu, H. Y., & Yu, Y. (2013). Lipid-rich bioalgal biomass production and nutrient removal by Haematococcus pluvialis in domestic secondary effluent. Ecological Engineering, 60, 155–159.
Yan, L., Schenk, P.M., (2011). Selection of cultured microalgae for producing omega-3 bio-lipid oil; report for Queensland Sea Scallop Trading Ltd. The University of Queensland, Queensland, Australia. 36.
Zhu, C. J., & Lee, Y. K. (1997). Determination of biomass dry weight of marine microalgae. Journal of Applied Phycology, 9, 189–194.
Acknowledgment
The authors sincerely acknowledge the Microbial Biotechnology Laboratory, University Institute of Engineering and Technology, M.D. University, Rohtak, Haryana, India for providing the laboratory facility for this research study.
Ethical Statement
The authors confirm that the present manuscript complies with the ethical rules applicable for this journal.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Dubey, K.K., Kumar, S., Dixit, D. et al. Implication of Industrial Waste for Biomass and Lipid Production in Chlorella minutissima Under Autotrophic, Heterotrophic, and Mixotrophic Grown Conditions. Appl Biochem Biotechnol 176, 1581–1595 (2015). https://doi.org/10.1007/s12010-015-1663-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-015-1663-6