Abstract
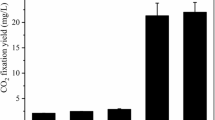
Five autotrophic strains isolated from non-photosynthetic microbial communities (NPMCs), which were screened from oceans with high CO2 fixing capability, were identified as Ochrobactrum sp. WH-2, Stenotrophomonas sp. WH-11, Ochrobactrum sp. WH-13, Castellaniella sp. WH-14, and Sinomicrobium oceani WH-15. The CO2 fixation pathways of all these strains were Calvin-Benson-Bassham pathway. These strains could metabolize multifarious organic compounds, which allowed switching them to autotrophic culture after enrichment in heterotrophic culture. The central composite response surface method indicated that these strains possessed many interactive effects, which increased the CO2 fixing efficiency of a combined community composed of these strains by 56 %, when compared with that of the single strain. Furthermore, another combined community composed of these autotrophic strains and NPMC had richer interactive relationships, with CO2 fixing efficiency being 894 % higher than that of the single strain and 148 % higher than the theoretical sum of the CO2 fixing efficiency of each of its microbial components. The interaction between strictly heterotrophic bacteria in NPMC and isolated autotrophic strains played a crucial role in improving the CO2 fixing efficiency, which not only eliminated self-restraint of organic compounds generated during the growth of autotrophic bacteria but also promoted its autotrophic pathway.




Similar content being viewed by others
References
Lopez, J. C., Quijano, G., Souza, T. S. O., Estrada, J. M., Lebrero, R., & Munoz, R. (2013). Biotechnologies for greenhouse gases (CH4, N2O, and CO2) abatement: state of the art and challenges. Applied Microbiology and Biotechnology, 97, 2277–2303.
Berg, I. A. (2011). Ecological aspects of the distribution of different autotrophic CO2 fixation pathways. Applied and Environmental Microbiology, 77, 1925–1936.
Falkowski, P., Scholes, R. J., Boyle, E., Canadell, J., Canfield, D., Elser, J., Gruber, N., Hibbard, K., Högberg, P., Linder, S., Mackenzie, F. T., Moore, B., III, Pedersen, T., Rosenthal, Y., Seitzinger, S., Smetacek, V., & Steffen, W. (2000). The global carbon cycle: a test of our knowledge of earth as a system. Science, 290, 291–296.
Acien, F., González-López, C. V., Fernández, J. M., & Molina, E. (2012). Conversion of CO2 into biomass by microalgae: how realistic a contribution may it be to significant CO2 removal? Applied Microbiology and Biotechnology, 96, 577–586.
Hopkinson, B. M., Dupontb, C. L., Allenb, A. E., & Morel, F. M. M. (2011). Efficiency of the CO2-concentrating mechanism of diatoms. Proceedings of the National academy of Sciences of the United States of America, 108, 3830–3837.
ElMekawy, A., Hegab, H. M., Vanbroekhoven, K., & Pant, D. (2014). Techno-productive potential of photosynthetic microbial fuel cells through different configurations. Renewable and Sustainable Energy Reviews, 39, 617–627.
Wu, X. H., Ge, T. D., Yuan, H. Z., Li, B. Z., Zhu, H. H., Zhou, P., Sui, F. G., O’Donnell, A. G., & Wu, J. S. (2014). Changes in bacterial CO2 fixation with depth in agricultural soils. Applied Microbiology and Biotechnology, 98, 2309–2319.
Madigan, M. T., Martinko, J. M., Stahl, D., & Clark, D. P. (2010). Brock biology of microorganisms (13th ed.). New York: Pearson Higher Education.
Herbert, R. A., Ranchou-Peyruse, A., Duran, R., Guyoneaud, R., & Schwabe, S. (2005). Characterization of purple sulfur bacteria from the South Andros Black Hole cave system: highlights taxonomic problems for ecological studies among the genera Allochromatium and Thiocapsa. Environmental Microbiology, 7, 1260–1268.
Li, B., Irvin, S., & Baker, K. (2007). The variation of nitrifying bacterial population sizes in a sequencing batch reactor (SBR) treating low, mid, high concentrated synthetic wastewater. Journal of Environmental Engineering and Science, 6, 651–663.
Alain, K., Querellou, J., Lesongeur, F., Pignet, P., Crassous, P., Raguénès, G., Cueff, V., & Cambon-Bonavita, M. A. (2002). Caminibacter hydrogeniphilus gen. nov., sp. nov., a novel thermophilic, hydrogen-oxidizing bacterium isolated from an East Pacific Rise hydrothermal vent. International Journal of Systematic and Evolutionary Microbiology, 52, 1317–1323.
Hu, J., Wang, L., Zhang, S., Fu, X., & Le, Y. (2010). Matching different inorganic compounds as mixture of electron donors to improve CO2 fixation by non-photosynthetic microbial community without hydrogen. Environmental Science and Technology, 44, 6364–6370.
Stams, A. J. M., & Plugge, C. M. (2009). Electron transfer in syntrophic communities of anaerobic bacteria and archaea. Nature Reviews Microbiology, 7, 568–577.
Hu, J., Wang, L., Zhang, S., Wang, Y., Jin, F., Fu, X., & Li, H. (2014). Universally improving effect of mixed electron donors on the CO2 fixing efficiency of non-photosynthetic microbial communities from marine environments. Journal of Environmental Sciences, 26, 1709–1716.
Tamura, K., Stecher, G., Peterson, D., Filipski, A., & Kumar, S. (2013). MEGA6: molecular evolutionary genetics analysis version 6.0. Molecular Biology and Evolution, 30, 2725–2729.
Tolli, J., & King, G. M. (2005). Diversity and structure of bacterial chemolithotrophic communities in pine forest and agroecosystem soils. Applied and Environmental Microbiology, 71, 8411–8418.
Kong, W., Dolhi, J. M., Chiuchiolo, A., Priscu, J., & Morgan-Kiss, R. M. (2012). Evidence of form II RubisCO (cbbM) in a perennially ice-covered Antarctic lake. FEMS Microbiology Ecology, 82, 491–500.
Takai, K., Campbell, B. J., Cary, S. C., Suzuki, M., Oida, H., Nunoura, T., Hirayama, H., Nakagawa, S., Suzuki, Y., Inagaki, F., & Horikoshi, K. (2005). Enzymatic and genetic characterization of carbon and energy metabolisms by deep-sea hydrothermal chemolithoautotrophic isolates of Epsilonproteobacteria. Applied and Environmental Microbiology, 71, 7310–7320.
Steinberg, L. M., & Regan, J. M. (2009). mcrA-targeted real-time quantitative PCR method to examine methanogen communities. Applied and Environmental Microbiology, 75, 4435–4442.
Romesburg, H. C. (2004). Cluster analysis for researchers. Raleigh: Lulu Press.
Maxwell, D., Pryor, F., & Smith, C. (2002). Cluster analysis in cross-sectional research. World Cultures, 13, 22–38.
Thauer, R. K. (2007). A fifth pathway of carbon fixation. Science, 14, 1732–1733.
Tabita, F. R., Hanson, T. E., Li, H., Satagopan, S., Singh, J., & Chan, S. (2007). Function, structure, and evolution of the RubisCO-like proteins and their RubisCO homologs. Microbiology and Molecular Biology Reviews, 71, 576–599.
Morris, B. E. L., Henneberger, R., Huber, H., & Moissl-Eichinger, C. (2013). Microbial syntrophy: interaction for the common good. FEMS Microbiology Ecology, 37, 384–406.
Nazaries, L., Murrell, J. C., Millard, P., Baggs, L., & Singh, B. K. (2013). Methane, microbes and models: fundamental understanding of the soil methane cycle for future predictions. Environmental Microbiology, 15, 2395–2417.
Schink, B. (1997). Energetics of syntrophic cooperation in methanogenic degradation. Microbiology and Molecular Biology Reviews, 61, 262–280.
Hu, J., Wang, L., Zhang, S., Wang, Y., & Xi, X. (2011). Inhibitory effect of organic carbon on CO2 fixing by non-photosynthetic microbial community isolated from the ocean. Bioresource Technology, 102, 7147–7153.
Nishihara, H., Igarashi, Y., Kodama, T., & Nakajima, T. (1993). Production and properties of glycogen in the marine obligate chemolithoautotroph, Hydrogenovibrio marinus. Journal of Fermentation and Bioengineering, 75, 414–416.
Nealson, K. H., & Saffarini, D. (1994). Iron and manganese in anaerobic respiration: environmental significance, physiology, and regulation. Annual Review of Microbiology, 48, 311–343.
Acknowledgments
The work was financially supported by the National Natural Science Foundation of China, No. 21177093 and No. 21307093; National High Technology Research and Development Program of China, No. 2012AA050101; Research Fund for the Doctoral Program of Higher Education of China, No. 20130072110025; China Postdoctoral Science Foundation, No. 2013M531220 and No. 2014T70430; Scientific Research Projects of Shanghai Science and Technology Committee, No. 14540500600; and Program for Professor of Special Appointment at Shanghai (Eastern Scholar) at Shanghai Institutions of Higher Learning.
Author information
Authors and Affiliations
Corresponding author
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
ESM 1
(DOCX 1331 kb)
Rights and permissions
About this article
Cite this article
Hu, J., Wang, L., Zhang, S. et al. Interactions Between Autotrophic and Heterotrophic Strains Improve CO2 Fixing Efficiency of Non-photosynthetic Microbial Communities. Appl Biochem Biotechnol 176, 1459–1471 (2015). https://doi.org/10.1007/s12010-015-1657-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-015-1657-4