Abstract
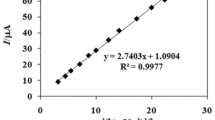
An electrochemical nucleic acid biosensor based on label-free DNA detection method was prepared for the first time by using electropolymerized poly(l-glutamic acid)-modified pencil graphite electrode (PGA/PGE) for detection of hepatitis C virus genotype 1a (HCV1a). Inosine-substituted 20-mer probes related to the HCV1a were immobilized onto PGA/PGE surface by covalent linking with the formation of amide bonds. Square wave voltammetry (SWV) was used to monitor the oxidation signal of guanine in the hybridization events, which gave an oxidation peak at +1.05 V. An increase in the oxidation signal of guanine was showed by hybridization of the probe with the complementary DNA. Noncomplementary oligonucleotides were also used to investigate the selectivity of the biosensor. The proposed nucleic acid biosensor was linear in the range of 50 nM to 1.0 μM, exhibiting a limit of detection of 40.6 nM. Finally, single-stranded synthetic PCR product analogues of HCV1a were performed in optimal condition. This PGA-modified nucleic acid sensor is cost-effective and disposable, and besides, it has superior electrocatalytic effect on the oxidation of guanine.








Similar content being viewed by others
References
Zhang, Y., Zhang, K., & Ma, H. (2009). Electrochemical DNA biosensors based on gold nanoparticles / cysteamine/ poly (glutamic acid) modified electrode. American Journal of Biomedical Sciences, 1(2), 115–125.
Qiu, L., Zhou, H., Wu, Z., Shen, G., & Yu, R. (2014). Sensitive and selective electrochemical DNA sensor for the analysis of cancer-related single nucleotide polymorphism. New Journal of Chemistry, 38(10), 4711–4715.
Benoit, V., Steel, A., Torres, M., Yu, Y.-Y., Yang, H., & Cooper, J. (2001). Evaluation of three-dimensional microchannel glass biochips for multiplexed nucleic acid fluorescence hybridization assays. Analytical Chemistry, 73(11), 2412–2420.
Barlet, V., Cohard, M., Thelu, M. A., Chaix, M. J., Baccard, C., Zarski, J. P., & Seigneurin, J. M. (1994). Quantitative detection of hepatitis B virus DNA in serum using chemiluminescence: comparison with radioactive solution hybridization assay. Journal of Virological Methods, 49(2), 141–151.
Kick, A., Bönsch, M., Katzschner, B., Voigt, J., Herr, A., Brabetz, W., Jung, M., Sonntag, F., Klotzbach, U., Danz, N., Howitz, S., & Mertig, M. (2010). DNA microarrays for hybridization detection by surface plasmon resonance spectroscopy. Biosensors & Bioelectronics, 26(4), 1543–1547.
Patolsky, F., Lichtenstein, A., & Willner, I. (2000). Amplified microgravimetric quartz-crystal-microbalance assay of DNA using oligonucleotide-functionalized liposomes or biotinylated liposomes. Journal of the American Chemical Society, 122(2), 418–419.
Dos Santos Riccardi, C., Kranz, C., Kowalik, J., Yamanaka, H., Mizaikoff, B., & Josowicz, M. (2008). Label-free DNA detection of hepatitis C virus based on modified conducting polypyrrole films at microelectrodes and atomic force microscopy tip-integrated electrodes. Analytical Chemistry, 80(1), 237–245.
Lucarelli, F., Marrazza, G., Turner, A. P., & Mascini, M. (2004). Carbon and gold electrodes as electrochemical transducers for DNA hybridisation sensors. Biosensors and Bioelectronics, 19(6), 515–530.
Chung, D.-J., Kim, K.-C., & Choi, S.-H. (2011). Electrochemical DNA biosensor based on avidin–biotin conjugation for influenza virus (type A) detection. Applied Surface Science, 257(22), 9390–9396.
Shakoori, Z., Salimian, S., Kharrazi, S., Adabi, M., & Saber, R. (2015). Electrochemical DNA biosensor based on gold nanorods for detecting hepatitis B virus. Analytical and Bioanalytical Chemistry, 407, 455–461.
Kashish, Soni, D. K., Mishra, S. K., Prakash, R., & Dubey, S. K. (2015). Label-free impedimetric detection of Listeria monocytogenes based on poly-5-carboxy indole modified ssDNA probe. Journal of Biotechnology, 200, 70–76.
Santos, D. P., Zanoni, M. V. B., Bergamini, M. F., Chiorcea-Paquim, A.-M., Diculescu, V. C., & Oliveira Brett, A.-M. (2008). Poly(glutamic acid) nanofibre modified glassy carbon electrode: characterization by atomic force microscopy, voltammetry and electrochemical impedance. Electrochimica Acta, 53(11), 3991–4000.
Zhang, Y., Luo, L., Ding, Y., Liu, X., & Qian, Z. (2010). A highly sensitive method for determination of paracetamol by adsorptive stripping voltammetry using a carbon paste electrode modified with nanogold and glutamic acid. Microchimica Acta, 171(1–2), 133–138.
Lin, Y., Liu, K., Liu, C., Yin, L., Kang, Q., Li, L., & Li, B. (2014). Electrochemical sensing of bisphenol A based on polyglutamic acid/amino-functionalised carbon nanotubes nanocomposite. Electrochimica Acta, 133, 492–500.
Ashraf-Uz-Zaman, M., Begum, B. A., Asad, H. B., Moutoshi, S. S., & Nasiruddin, M. (2010). Biochemical parameters in common viral hepatitis. Journal of Medicine, 11, 42–45.
Tahaei, S. M. E., Mohebbi, S. R., & Zali, M. R. (2012). Enteric hepatitis viruses. Gastroenterology and Hepatology From Bed to Bench, 5, 7–15.
World Health Organization (1999). Global surveillance and control of hepatitis C: Report of a WHO Consultation organized in collaboration with the Viral Hepatitis Prevention Board, Antwerp, Belgium. Journal of Viral Hepatitis, 6(1), 35–47. doi:10.1046/j.1365-2893.1999.6120139.x.
Consensus Statement (1999). EASL international consensus conference on hepatitis C. Journal of Hepatology, 30(5), 956–961. doi:10.1016/S0168-8278(99)80154-8.
Erensoy, S. (2001). Diagnosis of hepatitis C virus (HCV) infection and laboratory monitoring of its therapy. Journal of Clinical Virology, 21(3), 271–281.
Zhao, H., & Ju, H. (2004). Biosensor for hepatitis B virus DNA PCR product and electrochemical study of the interaction of Di(2,2′-bipyridine)osmium(III) with DNA. Electroanalysis, 16(19), 1642–1646.
Mandong, G., Yanqing, L., Hongxia, G., Xiaoqin, W., & Lifang, F. (2007). Electrochemical detection of short sequences related to the hepatitis B virus using MB on chitosan-modified CPE. Bioelectrochemistry, 70(2), 245–249.
Hejazi, M. S., Pournaghi-Azar, M. H., & Ahour, F. (2010). Electrochemical detection of short sequences of hepatitis C 3a virus using a peptide nucleic acid-assembled gold electrode. Analytical Biochemistry, 399(1), 118–124.
Pournaghi-Azar, M. H., Ahour, F., & Hejazi, M. S. (2009). Differential pulse voltammetric detection of hepatitis C virus 1a oligonucleotide chain by a label-free electrochemical DNA hybridization biosensor using consensus sequence of hepatitis C virus 1a probe on the pencil graphite electrode. Electroanalysis, 21(16), 1822–1828.
Kara, P., Cavdar, S., Meric, B., Erensoy, S., & Ozsoz, M. (2007). Electrochemical probe DNA design in PCR amplicon sequence for the optimum detection of microbiological diseases. Bioelectrochemistry (Amsterdam, Netherlands), 71(2), 204–210.
Wang, J., & Kawde, A.-N. (2002). Amplified label-free electrical detection of DNA hybridization. The Analyst, 127(3), 383–386.
Yu, A. M., & Chen, H. Y. (1997). Electrocatalytic oxidation of hydrazine at the poly(glutamic acid) chemically modified electrode and its amperometric determination. Analytical Letters, 30(3), 599–607.
Jiang, C., Yang, T., Jiao, K., & Gao, H. (2008). A DNA electrochemical sensor with poly-l-lysine/single-walled carbon nanotubes films and its application for the highly sensitive EIS detection of PAT gene fragment and PCR amplification of NOS gene. Electrochimica Acta, 53(6), 2917–2924.
Ma, W., & Sun, D. (2007). Electrocatalytical oxidation of dopamine and epinephrine at poly(L-threonine) modified electrode. Acta Physico-Chimica Sinica, 23(3), 332–336.
Liu, X., Luo, L., Ding, Y., & Ye, D. (2011). Poly-glutamic acid modified carbon nanotube-doped carbon paste electrode for sensitive detection of L-tryptophan. Bioelectrochemistry (Amsterdam, Netherlands), 82(1), 38–45.
Watterson, J. H., Piunno, P. A. E., Wust, C. C., & Krull, U. J. (2000). Effects of oligonucleotide immobilization density on selectivity of quantitative transduction of hybridization of immobilized DNA. Langmuir, 16(11), 4984–4992.
Kara, P., Cavusoglu, C., Cavdar, S., & Ozsoz, M. (2009). Direct electrochemical genosensing for multiple point mutation detection of Mycobacterium tuberculosis during the development of rifampin resistance. Biosensors and Bioelectronics, 24(6), 1796–1800.
Wu, D., Yin, B.-C., & Ye, B.-C. (2011). A label-free electrochemical DNA sensor based on exonuclease III-aided target recycling strategy for sequence-specific detection of femtomolar DNA. Biosensors and Bioelectronics, 28(1), 232–238.
Ricci, F., Bonham, A. J., Mason, A. C., Reich, N. O., & Plaxco, K. W. (2009). Reagentless, electrochemical approach for the specific detection of double- and single-stranded DNA binding proteins. Analytical Chemistry, 81(4), 1608–1614.
Farabullini, F., Lucarelli, F., Palchetti, I., Marrazza, G., & Mascini, M. (2007). Disposable electrochemical genosensor for the simultaneous analysis of different bacterial food contaminants. Biosensors and Bioelectronics, 22(7), 1544–1549.
Li, X. M., Ju, H. Q., Du, L. P., & Zhang, S. S. (2007). A nucleic acid biosensor for the detection of a short sequence related to the hepatitis B virus using bis(benzimidazole)cadmium(II) dinitrate as an electrochemical indicator. Journal of Inorganic Biochemistry, 101(8), 1165–1171.
Abad-Valle, P., Fernández-Abedul, M. T., & Costa-García, A. (2007). DNA single-base mismatch study with an electrochemical enzymatic genosensor. Biosensors and Bioelectronics, 22(8), 1642–1650.
Zhang, Q. D., March, G., Noel, V., Piro, B., Reisberg, S., Tran, L. D., & Pham, M. C. (2012). Label-free and reagentless electrochemical detection of PCR fragments using self-assembled quinone derivative monolayer: application to Mycobacterium tuberculosis. Biosensors and Bioelectronics, 32(1), 163–168.
Lai, R. Y., Seferos, D. S., Heeger, A. J., Bazan, G. C., & Plaxco, K. W. (2006). Comparison of the signaling and stability of electrochemical DNA sensors fabricated from 6- or 11-carbon self-assembled monolayers. Langmuir, 22(25), 10796–10800.
Pournaghi-Azar, M. H., Ahour, F., & Hejazi, M. S. (2010). Direct detection and discrimination of double-stranded oligonucleotide corresponding to hepatitis C virus genotype 3a using an electrochemical DNA biosensor based on peptide nucleic acid and double-stranded DNA hybridization. Analytical and Bioanalytical Chemistry, 397(8), 3581–3587.
Lucarelli, F., Marrazza, G., Palchetti, I., Cesaretti, S., & Mascini, M. (2002). Coupling of an indicator-free electrochemical DNA biosensor with polymerase chain reaction for the detection of DNA sequences related to the apolipoprotein E. Analytica Chimica Acta, 469(1), 93–99.
Huang, K.-J., Niu, D.-J., Sun, J.-Y., Han, C.-H., Wu, Z.-W., Li, Y.-L., & Xiong, X.-Q. (2011). Novel electrochemical sensor based on functionalized graphene for simultaneous determination of adenine and guanine in DNA. Colloids and Surfaces B: Biointerfaces, 82(2), 543–549.
Author information
Authors and Affiliations
Corresponding author
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
ESM 1
(DOC 183 kb)
Rights and permissions
About this article
Cite this article
Donmez, S., Arslan, F. & Arslan, H. A Nucleic Acid Biosensor for Detection of Hepatitis C Virus Genotype 1a Using Poly(l-Glutamic Acid)-Modified Electrode. Appl Biochem Biotechnol 176, 1431–1444 (2015). https://doi.org/10.1007/s12010-015-1655-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-015-1655-6