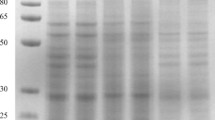
Abstract
Cell wall proteins (CWPs) are a prime site for signal perception and defense responses to environmental stresses. To gain further insights into CWPs and their molecular function, traditional techniques (e.g., two-dimensional gel electrophoresis) may be ineffective for special proteins. Elsholtzia splendens is a copper-tolerant plant species that grow on copper deposits. In this study, a fourplex isobaric tag was used for relative and absolute quantitation with liquid chromatography–tandem mass spectrometry approach to analyze the root CWPs of E. splendens. A total of 479 unique proteins were identified, including 121 novel proteins. Approximately 80.79 % of the proteins were extracted in the CaCl2 fraction, 16.08 % were detected in the NaCl fraction, and 3.13 % were identified in both fractions. The identified proteins have been involved in various processes, including cell wall remodeling, signal transduction, defense, and carbohydrate metabolism, thereby indicating a complex regulatory network in the apoplast of E. splendens roots. This study presents the first large-scale analysis of CWPs in metal-tolerant plants, which may be of paramount importance to understand the molecular functions and metabolic pathways in the root cell wall of copper-tolerant plants.






Similar content being viewed by others
References
Jarvis, M. C. (2009). Plant cell walls: supramolecular assembly, signalling and stress. Structural Chemistry, 20(2), 245–253.
Krzesłowska, M. (2011). The cell wall in plant cell response to trace metals: polysaccharide remodeling and its role in defense strategy. Acta Physiologiae Plantarum, 33(1), 35–51.
Zhu, J., Chen, S., Alvarez, S., Asirvatham, V. S., Schachtman, D. P., Wu, Y., & Sharp, R. E. (2006). Cell wall proteome in the maize primary root elongation zone. I. Extraction and identification of water-soluble and lightly ionically bound proteins. Plant Physiology, 140(1), 311–325.
Jamet, E., Albenne, C., Boudart, G., Irshad, M., Canut, H., & Pont‐Lezica, R. (2008). Recent advances in plant cell wall proteomics. Proteomics, 8(4), 893–908.
Held, M. A., Tan, L., Kamyab, A., Hare, M., Shpak, E., & Kieliszewski, M. J. (2004). Di-isodityrosine is the intermolecular cross-link of isodityrosine-rich extensin analogs cross-linked in vitro. Journal of Biological Chemistry, 279(53), 55474–55482.
Boudart, G., Jamet, E., Rossignol, M., Lafitte, C., Borderies, G., Jauneau, A., Esquerré‐Tugayé, M. T., & Pont‐Lezica, R. (2005). Cell wall proteins in apoplastic fluids of Arabidopsis thaliana rosettes: identification by mass spectrometry and bioinformatics. Proteomics, 5(1), 212–221.
Jamet, E., Boudart, G., Borderies, G., Charmont, S., Lafitte, C., Rossignol, M., Canut, H., & Pont-Lezica, R. (2008). In methods in molecular biology. vol. 2: 2D PAGE: Sample Preparation and Fractionation, Humana, Munich, Germany, pp. 187-201.
Dani, V., Simon, W. J., Duranti, M., & Croy, R. R. (2005). Changes in the tobacco leaf apoplast proteome in response to salt stress. Proteomics, 5(3), 737–745.
Soares, N. C., Francisco, R., Vielba, J. M., Ricardo, C. P., & Jackson, P. A. (2009). Associating wound-related changes in the apoplast proteome of Medicago with early steps in the ROS signal-transduction pathway. Journal of Proteome Research, 8(5), 2298–2309.
Jamet, E., Canut, H., Boudart, G., & Pont-Lezica, R. F. (2006). Cell wall proteins: a new insight through proteomics. Trends in Plant Science, 11(1), 33–39.
Bhushan, D., Pandey, A., Choudhary, M. K., Datta, A., Chakraborty, S., & Chakraborty, N. (2007). Comparative proteomics analysis of differentially expressed proteins in chickpea extracellular matrix during dehydration stress. Molecular & Cellular Proteomics, 6(11), 1868–1884.
Pandey, A., Rajamani, U., Verma, J., Subba, P., Chakraborty, N., Datta, A., Chakraborty, S., & Chakraborty, N. (2010). Identification of extracellular matrix proteins of rice (Oryza sativa L.) involved in dehydration-responsive network: a proteomic approach. Journal Proteome Research, 9(7), 3443–3464.
Floerl, S., Druebert, C., Majcherczyk, A., Karlovsky, P., Kües, U., & Polle, A. (2008). Defence reactions in the apoplastic proteome of oilseed rape (Brassica napus var. napus) attenuate Verticillium longisporum growth but not disease symptoms. BMC Plant Biology, 8(1), 129.
Goulet, C., Goulet, C., Goulet, M. C., & Michaud, D. (2010). 2‐DE proteome maps for the leaf apoplast of Nicotiana benthamiana. Proteomics, 10(13), 2536–2544.
Irshad, M., Canut, H., Borderies, G., Pont-Lezica, R., & Jamet, E. (2008). A new picture of cell wall protein dynamics in elongating cells of Arabidopsis thaliana: confirmed actors and newcomers. BMC Plant Biology, 8(1), 94.
Chivasa, S., Ndimba, B. K., Simon, W. J., Robertson, D., Yu, X. L., Knox, J. P., Bolwell, P., & Slabas, A. R. (2002). Proteomic analysis of the Arabidopsis thaliana cell wall. Electrophoresis, 23(11), 1754–1765.
Borderies, G., Jamet, E., Lafitte, C., Rossignol, M., Jauneau, A., Boudart, G., Monsarrat, B., Esquerré‐Tugayé, M. T., Boudet, A., & Pont‐Lezica, R. (2003). Proteomics of loosely bound cell wall proteins of Arabidopsis thaliana cell suspension cultures: a critical analysis. Electrophoresis, 24(19‐20), 3421–3432.
Albenne, C., Canut, H., & Jamet, E. (2013). Plant cell wall proteomics: the leadership of Arabidopsis thaliana. Frontiers in Plant Science, 4, 1–13.
Valente, M. A. S., Faria, J. A., Soares-Ramos, J. R., Reis, P. A., Pinheiro, G. L., Piovesan, N. D., Morais, A. T., Menezes, C. C., Cano, M. A., & Fietto, L. G. (2009). The ER luminal binding protein (BiP) mediates an increase in drought tolerance in soybean and delays drought-induced leaf senescence in soybean and tobacco. Journal of Experimental Botany, 60(2), 533–546.
Chen, X. Y., Kim, S. T., Cho, W. K., Rim, Y., Kim, S., Kim, S. W., Kang, K. Y., Park, Z. Y., & Kim, J. Y. (2009). Proteomics of weakly bound cell wall proteins in rice calli. Journal of Plant Physiology, 166(7), 675–685.
Millar, D. J., Whitelegge, J. P., Bindschedler, L. V., Rayon, C., Boudet, A. M., Rossignol, M., Borderies, G., & Bolwell, G. P. (2009). The cell wall and secretory proteome of a tobacco cell line synthesising secondary wall. Proteomics, 9(9), 2355–2372.
Bhushan, D., Pandey, A., Chattopadhyay, A., Choudhary, M. K., Chakraborty, S., Datta, A., & Chakraborty, N. (2006). Extracellular matrix proteome of chickpea (Cicer a rietinum L.) illustrates pathway abundance, novel protein functions and evolutionary perspect. Journal of Proteome Research, 5(7), 1711–1720.
Witzel, K., Shahzad, M., Matros, A., Mock, H. P., & Mühling, K. H. (2011). Comparative evaluation of extraction methods for apoplastic proteins from maize leaves. Plant Methods, 7(1), 48–52.
Watson, B. S., Lei, Z., Dixon, R. A., & Sumner, L. W. (2004). Proteomics of Medicago sativa cell walls. Phytochemistry, 65(12), 1709–1720.
Soares, N. C., Francisco, R., Ricardo, C. P., & Jackson, P. A. (2007). Proteomics of ionically bound and soluble extracellular proteins in Medicago truncatula leaves. Proteomics, 7(12), 2070–2082.
Verdonk, J. C., Hatfield, R. D., & Sullivan, M. L. (2012). Proteomic analysis of cell walls of two developmental stages of alfalfa stems. Frontiers in Plant Science, 3, 1–13.
Day, A., Fénart, S., Neutelings, G., Hawkins, S., Rolando, C., & Tokarski, C. (2013). Identification of cell wall proteins in the flax (Linum usitatissimum) stem. Proteomics, 13(5), 812–825.
Wang, S. B., Hu, Q., Sommerfeld, M., & Chen, F. (2004). Cell wall proteomics of the green alga Haematococcus pluvialis (Chlorophyceae). Proteomics, 4(3), 692–708.
Frigeri, L. G., Radabaugh, T. R., Haynes, P. A., & Hildebrand, M. (2013). Identification of proteins from a cell wall fraction of the diatom thalassiosira pseudonana insights into silica structure formation. Molecular & Cellular Proteomics 2006, 5(1), 182–193.
Wu, W. W., Wang, G., Baek, S. J., & Shen, R. F. (2006). Comparative study of three proteomic quantitative methods, DIGE, cICAT, and iTRAQ, using 2D gel-or LC-MALDI TOF/TOF. Journal of Proteome Research, 5(3), 651–658.
Yang, M. J., Yang, X. E., & Römheld, V. (2002). Growth and nutrient composition of Elsholtzia splendens Nakai under copper toxicity. Journal of Plant Nutrition, 25(7), 1359–1375.
Lou, L., Shen, Z., & Li, X. (2004). The copper tolerance mechanisms of Elsholtzia haichowensis, a plant from copper-enriched soils. Environmental and Experimental Botany, 51(2), 111–120.
Harrison, S., Lepp, N., & Phipps, D. (1979). Uptake of Copper by excised roots II. Copper desorption from the free space. Zeitschrift für Pflanzenphysiologie, 94(1), 27–34.
Feiz, L., Irshad, M., Pont-Lezica, R. F., Canut, H., & Jamet, E. (2006). Evaluation of cell wall preparations for proteomics: a new procedure for purifying cell walls from Arabidopsis hypocotyls. Plant Methods, 2, 10.
Bradford, M. M. (1976). A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Analytical Biochemistry, 72(1), 248–254.
Honjoh, K. I., Mimura, A., Kuroiwa, E., Hagisako, T., Suga, K., Shimizu, H., Dubeu, R. S., Miyamoto, T., Hatano, S., & IIo, M. (2003). Purification and characterization of two isoforms of glucose 6-phosphate dehydrogenase (G6PDH) from Chlorella vulgaris C-27. Bioscience, Biotechnology, and Biochemistry, 67(9), 1888–1896.
Ye, J., Fang, L., Zheng, H., Zhang, Y., Chen, J., Zhang, Z., Wang, J., Li, S., Li, R., & Bolund, L. (2006). WEGO: a web tool for plotting GO annotations. Nucleic Acids Research, 34(suppl 2), W293–W297.
Kanehisa, M., & Goto, S. (2000). KEGG: kyoto encyclopedia of genes and genomes. Nucleic Acids Research, 28(1), 27–30.
Feiz, L., Irshad, M., Pont-Lezica, R. F., Canut, H., & Jamet, E. (2006). Evaluation of cell wall preparations for proteomics: a new procedure for purifying cell walls from Arabidopsis hypocotyls. Plant Methods, 2(1), 10.
Kwon, H. K., Yokoyama, R., & Nishitani, K. (2005). A proteomic approach to apoplastic proteins involved in cell wall regeneration in protoplasts of Arabidopsis suspension-cultured cells. Plant and Cell Physiology, 46(6), 843–857.
Xu, Z., Escamilla-Treviño, L., Zeng, L., Lalgondar, M., Bevan, D., Winkel, B., Mohamed, A., Cheng, C. L., Shih, M. C., & Poulton, J. (2004). Functional genomic analysis of Arabidopsis thaliana glycoside hydrolase family 1. Plant Molecular Biology, 55(3), 343–367.
Narula, K., Elagamey, E., Datta, A., Chakraborty, N., Chakraborty, S., & Goyal, A. (2012). Comparative analyses of extracellular matrix proteome: an under-explored area in plant research. Crop Plants 7, 145-166.
Derbyshire, P., Findlay, K., McCann, M. C., & Roberts, K. (2007). Cell elongation in Arabidopsis hypocotyls involves dynamic changes in cell wall thickness. Journal of Experimental Botany, 58(8), 2079–2089.
Kevil, C. G., De Benedetti, A., Payne, D. K., Coe, L. L., Laroux, F. S., & Alexander, J. S. (1996). Translational regulation of vascular permeability factor by eukaryotic initiation factor 4E: implications for tumor angiogenesis. International Journal of Cancer, 65(6), 785–790.
Lu, P., Vogel, C., Wang, R., Yao, X., & Marcotte, E. M. (2006). Absolute protein expression profiling estimates the relative contributions of transcriptional and translational regulation. Nature Biotechnology, 25(1), 117–124.
Naujokat, C., & Hoffmann, S. (2002). Role and function of the 26S proteasome in proliferation and apoptosis. Laboratory Investigation, 82(8), 965–980.
Eikmanns, B. J., Thum-Schmitz, N., Eggeling, L., Lüdtke, K. U., & Sahm, H. (2013). Nucleotide sequence, expression and transcriptional analysis of the Corynebacterium glutamicum gltA gene encoding citrate synthase. Microbiology, 1994, 140(8), 1817–1828.
Jackson, R. J., Hellen, C., & Pestova, T. V. (2010). The mechanism of eukaryotic translation initiation and principles of its regulation. Nature Reviews Molecular Cell Biology, 11(2), 113–127.
Hor, L., Dobson, R. C., Downton, M. T., Wagner, J., Hutton, C. A., & Perugini, M. A. (2013). Dimerization of bacterial diaminopimelate epimerase is essential for catalysis. Journal of Biological Chemistry, 288(13), 9238–9248.
Xu, S., Butkevich, A. N., Yamada, R., Zhou, Y., Debnath, B., Duncan, R., Zandi, E., Petasis, N. A., & Neamati, N. (2012). Discovery of an orally active small-molecule irreversible inhibitor of protein disulfide isomerase for ovarian cancer treatment. Proceedings of the National Academy of Sciences, 109(40), 16348–16353.
Richard, J. P. (2012). A paradigm for enzyme-catalyzed proton transfer at carbon: triosephosphate isomerase. Biochemistry, 51(13), 2652–2661.
Chivasa, S., Ndimba, B. K., Simon, W. J., Lindsey, K., & Slabas, A. R. (2005). Extracellular ATP functions as an endogenous external metabolite regulating plant cell viability. The Plant Cell Online, 17(11), 3019–3034.
Ndimba, B. K., Chivasa, S., Hamilton, J. M., Simon, W. J., & Slabas, A. R. (2003). Proteomic analysis of changes in the extracellular matrix of Arabidopsis cell suspension cultures induced by fungal elicitors. Proteomics, 3(6), 1047–1059.
Stock, J., Ninfa, A., & Stock, A. (1989). Protein phosphorylation and regulation of adaptive responses in bacteria. Microbiological Reviews, 53(4), 450.
Chivasa, S., Simon, W. J., Yu, X. L., Yalpani, N., & Slabas, A. R. (2005). Pathogen elicitor‐induced changes in the maize extracellular matrix proteome. Proteomics, 5(18), 4894–4904.
Kasai, H., & Aosaki, T. (1989). Modulation of Ca-channel current by an adenosine analog mediated by a GTP-binding protein in chick sensory neurons. Pflügers Archiv, 414(2), 145–149.
Schwacke, R., Schneider, A., Van der Graaff, E., Fischer, K., Catoni, E., Desimone, M., Frommer, W. B., Flügge, U. I., & Kunze, R. (2003). ARAMEMNON, a novel database for Arabidopsis integral membrane proteins. Plant Physiology, 131(1), 16–26.
Murti, K., Smith, H. T., & Fried, V. A. (1988). Ubiquitin is a component of the microtubule network. Proceedings of the National Academy of Sciences, 85(9), 3019–3023.
Gokulakannan, G. G., & Niehaus, K. (2010). Characterization of the Medicago truncatula cell wall proteome in cell suspension culture upon elicitation and suppression of plant defense. Journal of Plant Physiology, 167(18), 1533–1541.
Passardi, F., Penel, C., & Dunand, C. (2004). Performing the paradoxical: how plant peroxidases modify the cell wall. Trends in Plant Science, 9(11), 534–540.
Higuchi, M., Pischke, M. S., Mähönen, A. P., Miyawaki, K., Hashimoto, Y., Seki, M., Kobayashi, M., Shinozaki, K., Kato, T., & Tabata, S. (2004). In planta functions of the Arabidopsis cytokinin receptor family. Proceedings of the National Academy of Sciences of the United States of America, 101(23), 8821–8826.
Imin, N., Kerim, T., Rolfe, B. G., & Weinman, J. J. (2004). Effect of early cold stress on the maturation of rice anthers. Proteomics, 4(7), 1873–1882.
Zhang, H., Xia, Y., Wang, G., & Shen, Z. (2008). Excess copper induces accumulation of hydrogen peroxide and increases lipid peroxidation and total activity of copper–zinc superoxide dismutase in roots of Elsholtzia haichowensis. Planta, 227(2), 465–475.
Timperio, A. M., Egidi, M. G., & Zolla, L. (2008). Proteomics applied on plant abiotic stresses: role of heat shock proteins (HSP). Journal of Proteomics, 71(4), 391–411.
Abe, S., Sakai, M., Yagi, K., Hagino, T., Ochi, K., Shibata, K., & Davies, E. (2003). A Tudor protein with multiple SNc domains from pea seedlings: cellular localization, partial characterization, sequence analysis, and phylogenetic relationships. Journal of Experimental Botany, 54(384), 971–983.
Qu, Z. L., Zhong, N. Q., Wang, H. Y., Chen, A. P., Jian, G. L., & Xia, G. X. (2006). Ectopic expression of the cotton non-symbiotic hemoglobin gene GhHbd1 triggers defense responses and increases disease tolerance in Arabidopsis. Plant and Cell Physiology, 47(8), 1058–1068.
Doyle, V., Virji, S., & Crompton, M. (1999). Evidence that cyclophilin-A protects cells against oxidative stress. Biochemistry Journal, 341, 127–132.
Acknowledgments
This study was supported by the National Natural Science Foundation of China (41422107, 21177109), the Fundamental Research Funds for the Central Universities (2015XZZX004-15), and Zhejiang University K. P. Chao’s High Technology Development Foundation (2014RC011). The authors would like to express their deepest gratitude to Dongfeng Liu from the College of Agriculture and Biotechnology in Zhejiang University for her kind help during the experiments.
Conflicts of Interest
The authors declare no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Highlights
• First large-scale analysis of CWPs in metal-tolerant plants
• Enhancement of knowledge regarding complexity of apoplast proteins in metal-tolerant plants
• Clues for further functional research of target proteins associated with metal-tolerant plants
Electronic supplementary material
Below is the link to the electronic supplementary material.
Fig. S1
Workflow of the experimental design for iTRAQ labeling showing biological replicates. Cell wall proteins from CaCl2 extraction were labeled with 114 and 116, respectively; Cell wall proteins from NaCl extraction were labeled with 115 and 117, respectively (DOC 28 kb)
Table S1
Subcellular localization and GO terms corresponding to the biological process of the 479 root cell wall proteins (XLS 506 kb)
Table S2
Relative quantitative information in CaCl2- and NaCl-extracted protein samples using 4-plex iTRAQ technology (XLS 277 kb)
Table S3
Peptides from 479 unique proteins (XLS 1841 kb)
Table S4
A total of 479 CWPs were identified including 121 novel proteins (DOC 774 kb)
Rights and permissions
About this article
Cite this article
Liu, T., Huang, C., Shen, C. et al. Isolation and Analysis of Cell Wall Proteome in Elsholtzia splendens Roots Using ITRAQ with LC–ESI–MS/MS. Appl Biochem Biotechnol 176, 1174–1194 (2015). https://doi.org/10.1007/s12010-015-1638-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-015-1638-7