Abstract
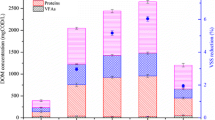
A representative thermophilic bacterial strain (AT06-1) capable of secreting protease was isolated from thermophilic aerobic digestion reactor, and 16S rRNA gene analysis indicated that it was Bacillus sp. The isolated strain was inoculated in waste activated sludge (WAS) to evaluate the performance of solubilization by thermophilic enzyme (S-TE) process under aerobic or microaerobic conditions at different temperatures (55–70 °C). Results showed that the inoculation of specific thermophilic strain significantly affected the volatile suspended solids (VSS) removal. At the optimal temperature of 65 °C, the maximum VSS removal of 43.6 % and highest SCOD of 4475 mg/L was achieved during microaerobic S-TE process. Compared to the noninoculation, more soluble protein was released during S-TE process due to the higher protease activity associated with the protein hydrolysis originated from cell lysis. The protease activity at aerobic and microaerobic S-TE process was respectively 1.73 and 1.88 times that of the noninoculation. Ammonia was the end nitrogenous compound of protein hydrolysis during S-TE process, which was stripped from the digestion system through continuous aeration.





Similar content being viewed by others
References
Christopher, J. R., & Nicholas, J. N. (1996). Pretreatment technology for the beneficial biological reuse of municipal sewage sludges. Applied Biochemistry and Biotechnology, 57–58, 983–991.
Egemen, E., Corpening, J., & Nirmalakhandan, N. (1994). Evaluation of an ozonation system for reduced waste sludge generation. Water Science and Technology, 44, 445–452.
Shiota, N., Akashi, A., & Hasegawa, S. (2002). A strategy in wastewater treatment process for significant reduction of excess sludge production. Water Science and Technology, 45, 127–134.
Tiehm, A., Nickel, K., Zellhorn, M., & Neis, U. (2001). Ultrasonic waste activated sludge disintegration for improving anaerobic stabilization. Water Research, 35, 2003–2009.
Mason, C. A., Häner, A., & Hamer, G. (1992). Aerobic thermophilic waste sludge treatment. Water Science and Technology, 25, 113–118.
Kim, Y. K., & Choi, J. W. (2002). Optimum operation of thermophilic aerobic digestion process for waste activated sludge minimization. Journal of Microbiology and Biotechnology, 12, 683–686.
Nakamichi, T., Nakashima, T., Fujisaki, H., Takamatsu, N., Muramatsu, T., Takahashi, Y., & Ishibashi, Y. (2010). Characteristics of Anoxybacillus sp. MU3 isolated from a hot spring and its application to the hyper thermal solubilization of sewage sludge. Environmental Engineering Science, 27, 993–999.
Layden, N. M., Mavinic, D. S., Kelly, H. G., Moles, R., & Bartlett, J. (2007). Autothermal thermophilic aerobic digestion (ATAD)—part I: review of origins, design, and process operation. Journal of Environmental Engineering and Science, 6, 665–678.
Hasegawa, S., Shiota, N., Katsura, K., & Akashi, A. (2000). Solubilization of organic sludge by thermophilic aerobic bacteria as a pretreatment for anaerobic digestion. Water Science and Technology, 41, 163–169.
Sakai, Y., Aoyagi, T., Shiota, N., Akashi, A., & Hasegawa, S. (2000). Complete decomposition of biological waste sludge by thermophilic aerobic bacteria. Water Science and Technology, 42, 81–88.
Kim, Y. K., Bae, J. H., Oh, B. K., Lee, W. H., & Choi, J. W. (2002). Enhancement of proteolytic enzyme activity excreted from Bacillus stearothermophilus for a thermophilic aerobic digestion process. Bioresource Technology, 82, 157–164.
Li, X. S., Ma, H. Z., Wang, Q. H., Matsumoto, S., Maeda, T., & Ogawa, H. I. (2009). Isolation, identification of sludge-lysing strain and its utilization in thermophilic aerobic digestion for waste activated sludge. Bioresource Technology, 100, 2475–2481.
Parmar, N., Singh, A., & Ward, O. P. (2001). Characterization of the combined effects of enzyme, pH and temperature treatments for removal of pathogens from sewage sludge. World Journal of Microbiology and Biotechnology, 17, 169–172.
Häner, A., Mason, C. A., & Hamer, G. (1994). Death and lysis during aerobic thermophilic sludge treatment: characterization of recalcitrant products. Water Research, 28, 863–869.
Kavitha, S., Adish Kumar, S., Yogalakshmi, K. N., Kaliappan, S., & Rajesh Banu, J. (2013). Effect of enzyme secreting bacterial pretreatment on enhancement of aerobic digestion potential of waste activated sludge interceded through EDTA. Bioresource Technology, 150, 210–219.
Weisburg, W. G., Barns, S. M., Pelletier, D. A., & Lane, D. J. (1991). 16S ribosomal DNA amplification for phylogenetic study. Journal of Bacteriology, 173, 697–703.
Eaton, A. D., Clesceri, L. S., Rice, E. W., Greenberg, A. E., & Franson, M. A. H. (2005). Standard methods for the examination of water and wastewater (21st ed.). Washington, DC: American Public Health Association.
Lowry, O. H., Rosebrough, N. J., Farr, A. L., & Randall, R. J. (1951). Protein measurement with the Folin phenol reagent. Journal of Biological Chemistry, 193, 265–275.
Fernadez, J., Perez, M., & Romer, L. I. (2010). Kinetics of mesophilic anaerobic digestion of the organic fraction of municipal solid waste: influence of initial total solid concentration. Bioresource Technology, 101, 6322–6328.
Mottet, A., Steyer, J. P., Vedrenne, F., Chauzy, J., & Carrer, H. (2009). Kinetics of thermophilic batch anaerobic digestion of thermal hydrolysed waste activated sludge. Biochemical Engineering Journal, 46, 169–175.
Yan, S. T., Miyanaga, K., Xing, X. H., & Tanji, Y. (2008). Succession of bacterial community and enzymatic activities of activated sludge by heat-treatment for reduction of excess sludge. Biochemical Engineering Journal, 39, 598–603.
Liu, S. G., Zhu, N. W., Li, L. Y., & Yuan, H. P. (2011). Isolation, identification and utilization of thermophilic strains in aerobic digestion of sewage sludge. Water Research, 45, 5959–5968.
Merrylin, J., Kaliappan, S., Adish Kumar, S., Yeom, I. T., & Rajesh Banu, J. (2014). Effect of extra polymeric substance removal on sludge reduction potential of Bacillus licheniformis at its optimised pH condition. Water Environment Journal, 28, 95–103.
Lakshmi, M. V., Merrylin, J., Kavitha, S., Adish Kumar, S., Rajesh Banu, J., & Yeom, I. T. (2014). Solubilization of municipal sewage waste activated sludge by novel lytic bacterial strains. Environmental Science and Pollution Research International, 21, 2733–2743.
Song, Y. D., & Hu, H. Y. (2006). Isolation and characterization of thermophilic bacteria capable of lysing microbial cells in activated sludge. Water Science and Technology, 54, 35–43.
Liu, S. G., Song, F. Y., Zhu, N. W., Yuan, H. P., & Cheng, J. H. (2010). Chemical and microbial changes during autothermal thermophilic aerobic digestion (ATAD) of sewage sludge. Bioresource Technology, 101, 9438–9444.
Kavitha, S., Adish Kumar, S., Kaliappan, S., Yeom, I. T., & Rajesh Banu, J. (2014). Improving the amenability of municipal waste activated sludge for biological pretreatment by phase-separated sludge disintegration method. Bioresource Technology, 169, 700–706.
Burgess, J. E., & Pletschke, B. I. (2008). Hydrolytic enzymes in sewage sludge treatment: a mini-review. Water SA, 34, 343–349.
Foladori, P., Andreottola, G., & Ziglio, G. (2010). Sludge reduction technologies in wastewater treatment plant. London: IWA.
Elliott, A., & Mahmood, T. (2007). Pretreatment technologies for advancing anaerobic digestion of pulp and paper biotreatment residues. Water Research, 41, 4273–4286.
Lee, J. W., Lee, H. W., Kim, S. W., Lee, S. Y., Park, Y. K., Han, J. H., Choi, S. I., Yi, Y. S., & Yun, Z. (2004). Nitrogen removal characteristics analyzed with gas and microbial community in thermophilic aerobic digestion for piggery waste treatment. Water Science and Technology, 49, 349–357.
Acknowledgments
This research was financially supported by the National Natural Science Foundation of China (51378188 and 51278175), the Doctoral Fund of Ministry of Education of China (20130161120021), the Planned Science and Technology Project of Changsha (k1407019-11), and the Young Teacher Growth Program of Hunan University.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yang, Q., Luo, K., Li, Xm. et al. Solubilization of Waste Activated Sludge and Nitrogenous Compounds Transformation During Solubilization by Thermophilic Enzyme (S-TE) Process. Appl Biochem Biotechnol 176, 700–711 (2015). https://doi.org/10.1007/s12010-015-1605-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-015-1605-3