Abstract
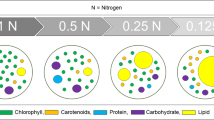
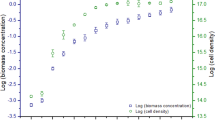
Microalgae have been proposed as a potential feedstock for biofuel production; however, cell disruption is usually required for collection and utilization of cytoplasmic polysaccharides and lipids. Virus infection might be one approach to disrupt the cell wall. The concentration of yeast extract and presence of KNO3 in algae cultivation media were investigated to observe their effects on Chlorella variabilis NC64A physiology and composition and the subsequent effect on production of Chlorella virus and disruption of infected cells. Cytoplasmic starch accumulation increased from 5 % to approximately 35 % of the total dry weight when yeast extract decreased from 1 to 0.25 g L−1. When cells were cultured with the lowest nitrogen levels, the total polysaccharide accounted for more than 50 % of the cell wall, which was 1.7 times higher than the content in cells cultured with the highest nitrogen levels. The C/N ratio of the algal biomass decreased by a factor of approximately 2 when yeast extract increased from 0.25 to 1 g L−1. After virus infection, cells with a low C/N ratio produced a 7.6 times higher burst size than cells with a high C/N ratio, suggesting that the nitrogen content in C. variabilis has a large influence on viral production and cell lysis. The results have implications on management of nitrogen for both the synthesis of products from algae and product recovery via viral lysis.




Similar content being viewed by others
References
Ball, S., Marianne, T., Dirick, L., Fresnoy, M., Delrue, B., & Decq, A. (1991). A Chlamydomonas reinhardtii low-starch mutant is defective for 3-phosphoglycerate activation and orthophosphate inhibition of ADP-glucose pyrophosphorylase. Planta, 185(1), 17–26.
Breitbart, M., & Rohwer, F. (2005). Here a virus, there a virus, everywhere the same virus? Trends in Microbiology, 13(6), 278–284. doi:10.1016/j.tim.2005.04.003.
Brennan, L., & Owende, P. (2010). Biofuels from microalgae—a review of technologies for production, processing, and extractions of biofuels and co-products. Renewable and Sustainable Energy Reviews, 14(2), 557–577. doi:10.1016/j.rser.2009.10.009.
Burgin, A. J., & Hamilton, S. K. (2007). Have we overemphasized the role of denitrification in aquatic ecosystems? A review of nitrate removal pathways. Frontiers in Ecology and the Environment, 5(2), 89–96. doi:10.1890/1540-9295(2007)5[89:HWOTRO]2.0.CO;2.
Cheng, Y.-S., Zheng, Y., Labavitch, J. M., & VanderGheynst, J. S. (2013). Virus infection of Chlorella variabilis and enzymatic saccharification of algal biomass for bioethanol production. Bioresource Technology, 137, 326–331.
Cheng, Y.-S., Zheng, Y., & VanderGheynst, J. S. (2011). Rapid quantitative analysis of lipids using a colorimetric method in a microplate format. Lipids, 46(1), 95–103.
Chuchird, N., Hiramatsu, S., Sugimoto, I., Fujie, M., Usami, S., & Yamada, T. (2001). Digestion of Chlorella cells by chlorovirus-encoded polysaccharide degrading enzymes. Microbes and Environments, 16(4), 206–212.
Clasen, J. L., & Elser, J. J. (2007). The effect of host Chlorella NC64A carbon : phosphorus ratio on the production of Paramecium bursariaChlorella Virus1. Freshwater Biology, 52(1), 112–122. doi:10.1111/j.1365-2427.2006.01677.x.
Dallies, N., François, J., & Paquet, V. (1998). A new method for quantitative determination of polysaccharides in the yeast cell wall. Application to the cell wall defective mutants of Saccharomyces cerevisiae. Yeast, 14(14), 1297–1306.
Elser, J. J., Bracken, M. E., Cleland, E. E., Gruner, D. S., Harpole, W. S., Hillebrand, H., Ngai, J. T., Seabloom, E. W., Shurin, J. B., & Smith, J. E. (2007). Global analysis of nitrogen and phosphorus limitation of primary producers in freshwater, marine and terrestrial ecosystems. Ecology Letters, 10(12), 1135–1142. doi:10.1111/j.1461-0248.2007.01113.x.
Etten JLV (1999) World of Chlorella Viruses Home Page. PUblisher. http://www.pbcv.org/isolate.html.
Fuhrman, J. A. (1999). Marine viruses and their biogeochemical and ecological effects. Nature, 399(6736), 541–548.
Gilbert D (2010) The PRIMER. In: DOE (ed). vol 7. Joint Genome Institute, Walnut Creek, CA 94598, USA, p 1.
Hupfer, M., Gloess, S., & Grossart, H.-P. (2007). Polyphosphate-accumulating microorganisms in aquatic sediments. Aquatic Microbial Ecology, 47(3), 299–311. doi:10.3354/ame047299.
Jansson, M. (1988). Phosphate uptake and utilization by bacteria and algae. Hydrobiologia, 170(1), 177–189.
Kamako, S.-i., Hoshina, R., Ueno, S., & Imamura, N. (2005). Establishment of axenic endosymbiotic strains of Japanese Paramecium bursaria and the utilization of carbohydrate and nitrogen compounds by the isolated algae. European Journal of Protistology, 41(3), 193–202. doi:10.1016/j.ejop.2005.04.001.
Kapaun, E., Loos, E., & Reisser, W. (1992). Cell wall composition of virus-sensitive symbiotic Chlorella species. Phytochemistry, 31(9), 3103–3104.
Keck, K., & Stich, H. (1957). The widespread occurrence of polyphosphate in lower plants. Annals of Botany, 21(4), 611–619.
Kim, S.-H., Liu, K.-H., Lee, S.-Y., Hong, S.-J., Cho, B.-K., Lee, H., Lee, C.-G., & Choi, H.-K. (2013). Effects of light intensity and nitrogen starvation on glycerolipid, glycerophospholipid, and carotenoid composition in Dunaliella tertiolecta culture. PLoS ONE, 8(9), e72415. doi:10.1371/journal.pone.0072415.
Li, W., Gao, K., & Beardall, J. (2012). Interactive effects of ocean acidification and nitrogen-limitation on the diatom Phaeodactylum tricornutum. PLoS ONE, 7(12), e51590. doi:10.1371/journal.pone.0051590.
MacLean WC, Warwick P, Food and Agriculture Organization of the United Nations. (2003) Methods of food analysis Food energy : methods of analysis and conversion factors : report of a technical workshop, Rome, 3-6 December 2002. Food and Agriculture Organization of the United Nations, Rome, pp vi, 87 p.
Matros, A., Amme, S., Kettig, B., Buck-Sorlin, G. H., Sonnewald, U., & Mock, H. P. (2006). Growth at elevated CO2 concentrations leads to modified profiles of secondary metabolites in tobacco cv. Samsun NN and to increased resistance against infection with potato virus Y. Plant, Cell and Environment, 29(1), 126–137. doi:10.1111/j.1365-3040.2005.01406.x.
Meints, R. H., Burbank, D. E., Van Etten, J. L., & Lamport, D. T. A. (1988). Properties of the Chlorella receptor for the virus PBCV-11. Virology, 164(1), 15–21. doi:10.1016/0042-6822(88)90614-9.
Middelboe, M. (2000). Bacterial growth rate and marine virus–host dynamics. Microbial Ecology, 40(2), 114–124. doi:10.1007/s002480000050.
Molina Grima, E., Belarbi, E. H., Acién Fernández, F. G., Robles Medina, A., & Chisti, Y. (2003). Recovery of microalgal biomass and metabolites: process options and economics. Biotechnology Advances, 20(7–8), 491–515.
Peltier, G., & Schmidt, G. W. (1991). Chlororespiration: an adaptation to nitrogen deficiency in Chlamydomonas reinhardtii. Proceedings of the National Academy of Sciences of the United States of America, 88(11), 4791–4795.
Peverly, J. H., Adamec, J., & Parthasarathy, M. V. (1978). Association of potassium and some other monovalent cations with occurrence of polyphosphate bodies in Chlorella pyrenoidosa. Plant Physiology, 62(1), 120–126. doi:10.1104/pp. 62.1.120.
Rabalais, N. N. (2002). Nitrogen in aquatic ecosystems. Ambio, 31(2), 102–112.
Saux, C., Lemoine, Y., Marion-Poll, A., Valadier, M. H., Deng, M., & Morot-Gaudry, J. F. (1987). Consequence of absence of nitrate reductase activity on photosynthesis in Nicotiana plumbaginifolia plants. Plant Physiology, 84(1), 67–72. doi:10.1104/pp. 84.1.67.
Seifert, G. J. (2004). Nucleotide sugar interconversions and cell wall biosynthesis: how to bring the inside to the outside. Current Opinion in Plant Biology, 7(3), 277–284. doi:10.1016/j.pbi.2004.03.004.
Shurin, J. B., Abbott, R. L., Deal, M. S., Kwan, G. T., Litchman, E., McBride, R. C., Mandal, S., & Smith, V. H. (2013). Industrial-strength ecology: trade-offs and opportunities in algal biofuel production. Ecology Letters, 16(11), 1393–1404. doi:10.1111/ele.12176.
Suttle, C. A. (2005). Viruses in the sea. Nature, 437(7057), 356–361.
van den Hoogen, B. M., van Weeren, P. R., Lopes-Cardozo, M., van Golde, L. M. G., Barneveld, A., & van de Lest, C. H. A. (1998). A microtiter plate assay for the determination of uronic acids. Analytical Biochemistry, 257(2), 107–111.
Van den Koornhuyse, N., Libessart, N., Delrue, B., Zabawinski, C., Decq, A., Iglesias, A., Carton, A., Preiss, J., & Ball, S. (1996). Control of starch composition and structure through substrate supply in the monocellular alga Chlamydomonas reinhardtii. Journal of Biological Chemistry, 271(27), 16281–16287.
Van Etten, J. L. (2003). Unusual life style of giant Chlorella viruses. Annual Review of Genomics, 37(1), 153–195. doi:10.1146/annurev.genet.37.110801.143915.
Van Etten, J. L., Burbank, D. E., Xia, Y., & Meints, R. H. (1983). Growth cycle of a virus, PBCV-1, that infects Chlorella-like algae. Virology, 126(1), 117–125. doi:10.1016/0042-6822(83)90466-x.
Wilhelm S, Suttle C Viruses as regulators of nutrient cycles in aquatic environments. In: Microbial biosystems: new frontiers Proceedings of the VIII International Symposium on Microbial Ecology Atlantic Canada Society for Microbial Ecology, Halifax, Nova Scotia, 2000. p 551-556.
Wilhelm, S. W., & Suttle, C. A. (1999). Viruses and nutrient cycles in the sea. BioScience, 49(10), 781–788. doi:10.1525/bisi.1999.49.10.781.
Wommack, K. E., & Colwell, R. R. (2000). Virioplankton: viruses in aquatic ecosystems. Microbiology and Molecular Biology Reviews, 64(1), 69–114. doi:10.1128/mmbr. 64.1.69-114.2000.
Acknowledgments
This research was supported by the Chevron Technology Ventures. They had no involvement in the design of the study; in the collection, analysis, and interpretation of data; or in the writing of the article. They were involved in the decision to submit the article for publication.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Cheng, YS., Labavitch, J. & VanderGheynst, J.S. Organic and Inorganic Nitrogen Impact Chlorella variabilis Productivity and Host Quality for Viral Production and Cell Lysis. Appl Biochem Biotechnol 176, 467–479 (2015). https://doi.org/10.1007/s12010-015-1588-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-015-1588-0