Abstract
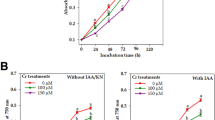
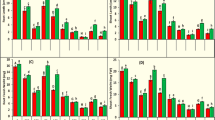
Siderophore production in Anabaena oryzae was investigated under the influence of various levels of iron and other abiotic factors such as pH, temperature, light and different nitrogen sources. Optimization of culture conditions under controlled mechanisms of these abiotic factors lead to the siderophore production in significant amount. Under iron-starved condition, A. oryzae extracellularly releases 89.17 % hydroxymate-type siderophore. Slightly alkaline pH and 30 °C temperature was found stimulatory for the cyanobacterial growth and siderophorogenesis (88.52 % SU and 83.87 % SU, respectively). Excess iron loading had a negative impact on siderophore production along with the alterations in the morphology and growth. Further, scanning electron microphotographs signified that higher concentrations of iron lead to complete damage of the cells and alterations in membrane proteins possibly transporters responsible for exchange of siderophore complex from environment to the cell. SDS-PAGE analysis of whole cell proteins showed overexpression of low molecular weight proteins ranges between 20.1 to 29.0 kDa up to 100-μM iron concentrations. These polypeptides/proteins might be involved in maintaining iron homeostasis by regulating siderophore production. Results suggest that lower concentrations of iron ≤50 μM along with other abiotic factors are stimulatory, whereas higher concentrations (>50 μM) are toxic. Data further suggested that cyanobacterium A. oryzae can serve as a potential biofertilizer especially in iron-rich soil through sequestration by the power of natural Fe(III)-siderophore complex formation.







Similar content being viewed by others
References
Brown, C. M., & Trick, C. G. (1992). Response of the cyanobacterium, Oscillatoria tenuis, to low iron environments: the effect on growth rate and evidence for siderophore production. Archives of Microbiology, 157, 349–354.
Singh, A., Mishra, A. K., Singh, S. S., Sarma, H. K., & Shukla, E. (2008). Influence of iron and chelator on siderophore production in Frankia strains nodulating Hippӧphae salicifolia D. Don. Journal of Basic Microbiology, 48, 104–111.
Page, M. G. P. (2013). Siderophore conjugates. Annals of the New York Academy of Sciences, 1277, 115–126.
Ferreira, F., & Straus, N. A. (1994). Iron deprivation in cyanobacteria. Journal of Applied Phycology, 6, 199–210.
Lammers, P. J., & Sanders-Loehr, J. (1982). Active transport of ferric schizokinen in Anabaena sp. Journal of Bacteriology, 151, 288–294.
Braun, V., Schäffer, S., Hantke, K., & Trӧger, W. (1990). Regulation of gene expression by Iron. In G. Hauska & R. Thauer (Eds.), The molecular basis of bacterial metabolism (pp. 164–179). New York, USA: Springer.
Winkelmann, G. (2002). Microbial siderophore-mediated transport. Biochemical Society Transactions, 30, 691–696.
Poole, K., & McKay, G. A. (2003). Iron acquisition and its control in Pseudomonas aeruginosa: many roads lead to Rome. Frontiers in Bioscience, 8, 661–686.
Lankford, C. E. (1973). Bacterial assimilation of iron. Critical Reviews in Microbiology, 2, 273–331.
Boukhalfa, H., & Crumbliss, A. L. (2002). Chemical aspects of siderophore mediated iron transport. Biometals, 15(4), 325–339.
Neilands, J. B. (1995). Siderophores structure and function of microbial iron transport compounds. Journal of Biological Chemistry, 270(8), 26723–26726.
Faraldo-Gomez, J. D., & Sansom, M. S. (2003). Acquisition of siderophores in gram-negative bacteria. Nature Reviews of Molecular Cell Biology, 4, 105–116.
Braun, V., & Hantke, K. (2000). Receptor-mediated bacterial iron transport in transition metals. In G. Winkelmann & C. J. Carrano (Eds.), Microbial metabolism (ch. 3) (pp. 81–116). London: Harwood.
Krewalak, K. D., Peacock, R. S., & Vogel, H. J. (2004). Periplasmic binding proteins involved in bacterial iron uptake. In J. H. Crosa, A. R. Mey, & S. M. Payne (Eds.), Ion transport in bacteria (ch. 8) (pp. 113–132). Washington: ASM Press.
Hider, R. C., & Kong, X. (2010). Chemistry and biology of siderophores. Natural Product Reports, 27, 637–657.
Postle, K., & Larsen, R. A. (2007). TonB-dependent energy transduction between outer and cytoplasmic membranes. Biometals, 20, 453–465.
Krewulak, K. D., & Vogel, H. J. (2008). TonB-dependent energy transduction between outer and cytoplasmic membranes. Biochimica et Biophysica Acta, 1778, 1781–1804.
Ratering, S., & Schnell, S. (2001). Nitrate-dependent iron(II) oxidation in paddy soil. Environmental Microbiology, 3(2), 100–109.
Whitton, B. A., & Whitton, B. A. (2000). Soils and rice fields. In M. Potts (Ed.), The ecology of cyanobacteria (ch 8) (pp. 233–255). Netherlands: Kluwer Academic Publishers.
Simpson, F. B., & Neilands, J. B. (1976). Siderochromes in cyanophyceae: isolation and characterization of schizokinen from Anabaena sp. Journal of Phycology, 12, 44–48.
Beiderbeck, H., Taraz, K., Budzikiewicz, H., & Walsby, A. E. (2000). Anachelin, the siderophore of the cyanobacterium Anabaena cylindrica CCAP 1403/2A. Zeitschrift fur Naturforschung C: Journal of Biosciences, 55, 681–687.
Castenholz, R. W. (2001). Phylum BX. Cyanobacteria, oxygenic photosynthetic bacteria. In D. R. Boone & R. W. Castenholz (Eds.), Bergey’s manual of systematic bacteriology (Vol. 1, pp. 473–597). New York: Springer.
Goldman, S. J., Lammers, P. J., Berman, M. S., & Sanders-Loehr, J. (1983). Siderophore-mediated iron uptake in different strains of Anabaena sp. Journal of Bacteriology, 156, 1144–1150.
Bertrand, S., Larcher, G., Landreau, A., Richomme, P., Duval, O., & Bouchara, J. (2009). Hydroxamate siderophores of Scedosporium apiospermum. Biometals, 22, 1019–1029.
Rippka, R., Deruelles, J., Waterbury, J. B., Herdman, M., & Stanier, R. Y. (1979). Generic assignments, strain histories and properties of pure cultures of cyanobacteria. Journal of General Microbiology, 111, 1–61.
Lowry, O. H., Rosebrough, N. J., Farr, A. L., & Randall, B. J. (1951). Protein measurements with the folin phenol reagent. Journal of Biological Chemistry, 193, 265–275.
Payne, S. M. (1994). Detection, isolation and characterization of siderophores. Methods in Enzymology, 235, 329–342.
Csaky, T. (1948). On the estimation of bound hydroxylamine in biological materials. Acta Chemica Scandinavica, 2, 240–454.
Atkin, C. L., Neilands, J. B., & Phaff, H. (1970). Rhodotorulic acid from species of Rhodospirillum, Rhodotorula, Sporidiobolus and Sporobolomyces. Journal of Bacteriology, 103, 722–733.
Arnow, L. E. (1937). Colorimetric determination of the components of 3, 4- dihydroxyphenylalanine tyrosine mixtures. Journal of Biological Chemistry, 118, 531–537.
Schwyn, B., & Neilands, J. B. (1987). Universal chemical assay for the detection and determination of siderophores. Analytical Biochemistry, 160, 47–56.
Mishra, A. K., Shukla, E., & Singh, S. S. (2013). Phylogenetic comparison among the heterocystous cyanobacteria based on a polyphasic approach. Protoplasma, 250, 77–94.
Santos, S., Neto, I. F. F., Machado, M. D., Soares, H. M. V. M., & Soares, E. V. (2014). Siderophore production by Bacillus megaterium: effect of growth phase and cultural conditions. Applied Biochemistry and Biotechnology, 172(1), 549–560.
Singh, A., Singh, S. S., Pandey, P. C., & Mishra, A. K. (2010). Attenuation of metal toxicity by frankial siderophores. Toxicological and Environmental Chemistry, 92, 1339–1346.
Ito, Y., & Butler, A. (2005). Structure of synechobactins, new siderophores of the marine cyanobacterium Synechococcus sp. PCC 7002. Limnology and Oceanography, 50, 1918–1923.
Sayyed, R. Z., Badgujar, M. D., Sonawane, H. M., Mhaske, M. M., & Chincholkar, S. B. (2005). Production of microbial iron chelators (Siderophores) by fluorescent Pseudomonads. Indian Journal of Biotechnology, 4, 484–490.
Ghassemian, M., & Straus, N. A. (1996). Fur regulates the expression of iron-stress genes in the cyanobacterium Synechococcus sp. Strain PCC 7942. Microbiology, 142, 1469–1476.
Hernández, J. A., López-Gomollón, S., Bes, M. T., Fillat, M. F., & Peleato, M. L. (2004). Three fur homologues from Anabaena sp. PCC7120: exploring reciprocal protein-promoter recognition. FEMS Microbiology Letters, 236, 275–282.
Hider, R. C. (1984). Siderophore mediated absorption of iron. Structure and Bonding, 58, 25–87.
Trick, C. G., & Kerry, A. (1992). Isolation and purification of siderophores produced by cyanobacteria, Synechococcus (Anacystis nidulans R2) sp. and Anabaena variabilis. Current Microbiology, 24, 24 l–245.
Chiadò, A., Varani, L., Bosco, F., & Marmo, L. (2013). Opening study on the development of a New biosensor for metal toxicity based on pseudomonas fluorescens pyoverdine. Biosensors, 3, 385–399.
Fallahzadeh, V., Ahmadzadeh, M., & Sharifi, R. (2010). Growth and pyoverdine production kinetics of Pseudomonas aeruginosa 7NSK2 in an experimental fermentor. Journal of Agricultural Technology, 6(1), 107–115.
Sunda, W., & Huntsman, S. (2003). Effect of pH, light and temperature on Fe-EDTA chelation and Fe hydrolysis in seawater. Marine Chemistry, 84, 35–47.
Rachid, D., & Ahmed, B. (2005). Effect of iron and growth inhibitors on siderophores production by Pseudomonas fluorescens. African Journal of Biotechnology, 4, 697–702.
Drechsel, H., & Jung, G. (1998). Peptide siderophores. Journal of Peptide Science, 4(3), 147–181.
McMillan, D. G. G., Velasquez, I., Nunn, B. L., Goodlett, D. R., Hunter, K. A., Lamont, I., Sander, S. G., & Cook, G. M. (2010). Acquisition of iron by alkaliphilic Bacillus species. Applied and Environmental Microbiology, 76(20), 6955–6961.
Hutchins, D. A., Rueter, J. G., & Fish, W. (1991). Siderophore production and nitrogen fixation are mutually exclusive strategies in Anabaena 7120. Limnology and Oceanography, 36, 1–12.
Kerry, A., Laudenbach, D. E., & Trick, C. G. (1988). Influence of iron limitation and nitrogen source on growth and siderophore production by cyanobacteria. Journal of Phycology, 24, 566–571.
Averil, B. A., & Orme-Johnson, W. H. (1978). Iron sulphur protein and their synthetic analogue In H. Siegal (Ed.), Metal in biological systems (pp 1387). New York: Marcel Dakker.
Kustka, A., Carpenter, E. J., & Sanudo-Wilhelmy, S. A. (2002). Iron and marine nitrogen fixation: Progress and future directions. Research in Microbiology, 153, 255–262.
Flores, E., & Herrero, A. (2005). Nitrogen assimilation and nitrogen control in cyanobacteria. Biochemical Society Transactions, 33(1), 164–167.
Barbeau, K., Zhang, G., Live, D. H., & Butler, A. (2002). Petrobactin, a photoreactive siderophore produced by the oil-degrading marine bacterium Marinobacter hydrocarbonoclasticus. Journal of the American Chemical Society, 124, 378–379.
Borer, P. M., Sulzberger, B., Reichard, P., & Kraemer, S. M. (2005). Effect of siderophores on light – induced dissolution of colloidal iron (III) (hydr)oxides. Marine Chemistry, 93, 179–193.
Barbeau, K. K., Rue, E. L., Trick, C. G., Bruland, K. W., & Butler, A. (2003). Photochemical reactivity of siderophores produced by marine heterotrophic bacteria and cyanobacteria based on characteristic Fe(III) binding groups. Limnology and Oceanography, 48, 1069–1078.
Amin, S. A., Green, D. H., Mark, H. C., Kupper, F. C., Sunda, W. G., & Carrano, C. J. (2009). Photolysis of iron-siderophore chelates promotes bacterial-algal mutualism. Proceedings of the National Academy of Sciences, 106, 17071–17076.
Murugappan, R. M., Karthikeyan, M., Aravinth, A., & Alamelu, M. R. (2012). Siderophore mediated iron uptake promotes yeast-bacterial symbiosis. Applied Biochemistry and Biotechnology, 168, 2170–2183.
Andrews, S. C., Robinson, A. K., & Rodríguez-Quiñones, F. (2003). Bacterial iron homeostasis. FEMS Microbiology Reviews, 27, 215–237.
Cornelis, P., Wei, Q., Andrews, S. C., & Vinckx, T. (2011). Iron homeostasis and management of oxidative stress response in bacteria. Metallomics, 3, 540–549.
Acknowledgments
We are very thankful to Dr. Alok Kumar Srivastava (Senior Scientist) and Mr. Manish Roy (Technical Assistant), National Bureau of Agriculturally Important Microorganisms, Mau nath Bhanjan, UP, India, for providing scanning electron microscopy facility. Central Instrumental Laboratory, Department of Botany, BHU for technical assistance in spectroscopic experiments. The Head, Department of Botany, BHU, Varanasi, India, is gratefully acknowledged for providing laboratory facilities.
Conflict of Interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Anumeha Singh, holds a MSc degree, Banaras Hindu University.
Dr. Arun Kumar Mishra, holds a PhD degree, is a Professor of Banaras Hindu University.
Rights and permissions
About this article
Cite this article
Singh, A., Mishra, A.K. Influence of Various Levels of Iron and Other Abiotic Factors on Siderophorogenesis in Paddy Field Cyanobacterium Anabaena oryzae . Appl Biochem Biotechnol 176, 372–386 (2015). https://doi.org/10.1007/s12010-015-1581-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-015-1581-7