Abstract
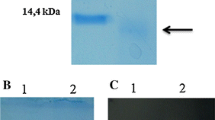
We present here the structural modeling and biochemical characterization of a recombinant superoxide dismutase (SOD) from Deschampsia antarctica E. Desv. [Poaceae] produced in Escherichia coli. The recombinant protein was purified by affinity chromatography nickel-nitrilotriacetic acid (Ni-NTA), and its identity was demonstrated by immunoblotting and inhibition by H2O2 and KCN. Inductively coupled plasma optical emission spectroscopy (ICP-OES) analysis confirmed the presence of Cu and Zn. Modeling of the D. antarctica Cu/Zn-SOD (DaSOD) amino acid sequence using the SWISS-MODEL and 2Q2L_B monomer of the psychrophilic Cu/Zu-SOD from Potentilla atrosanguinea (PaSOD) as template produced a structure similar to that of the typical eukaryotic Cu/Zn-SODs. Activity assays using the p-nitro blue tetrazolium chloride (NBT) solution method showed that the purified DaSOD had a specific activity of 5818 U/mg at 25 °C and pH 7.2 and that it was active in a pH interval of 5–8 and a temperature interval of 0–40 °C. Furthermore, DaSOD was still active at −20 °C as observed by a zymogram assay. We found 100 % activity when it was heated at 80 °C for 60 min, indicating a high thermostability. DaSOD properties suggest that this enzyme could be useful for preventing the oxidation of refrigerated or frozen foods, as well as in the preparation of cosmetic and pharmaceutical products.








Similar content being viewed by others
References
Bravo, L. A., Ulloa, N., Zuniga, G. E., Casanova, A., Corcuera, L. J., & Alberdi, M. (2001). Cold resistance in Antarctic angiosperms. Physiologiae Plantarum, 111, 55–65.
Perez-Torres, E., Garcia, A., Dinamarca, J., Alberdi, M., Gutierrez, A., Gidekel, M., Ivanov, A. G., Huner, N. P. A., Corcuera, L. J., & Bravo, L. A. (2004). The role of photochemical quenching and antioxidants in photoprotection of Deschampsia antarctica. Functional Plant Biology, 31, 731–741.
Fridovich, I. (1997). Superoxide anion radical (O-2 radical anion), superoxide dismutases, and related matters. Journal of Biological Chemistry, 272, 18515–18517.
Alscher, R. G., Erturk, N., & Heath, L. S. (2002). Role of superoxide dismutases (SODs) in controlling oxidative stress in plants. Journal of Experimental Botany, 53, 1331–1341.
Youn, H. D., Kim, E. J., Roe, J. H., Hah, Y. C., & Kang, S. O. (1996). A novel nickel-containing superoxide dismutase from Streptomyces spp. Biochemical Journal, 318, 889–896.
Richardson, J. S. (1977). beta-Sheet topology and the relatedness of proteins. Nature, 268, 495–500.
Bafana, A., Dutt, S., Kumar, S., & Ahuja, P. S. (2011). Superoxide dismutase: an industrial perspective. Critical Reviews in Biotechnology, 31, 65–76.
Di Mambro, V. M., & Fonseca, M. J. (2007). Assessment of physical and antioxidant activity stability, in vitro release and in vivo efficacy of formulations added with superoxide dismutase alone or in association with alpha-tocopherol. European Journal of Pharmaceutics and Biopharmaceutics, 66, 451–459.
Mizushima, Y., Hoshi, K., Yanagawa, A., & Takano, K. (1991). Topical application of superoxide dismutase cream. Drugs Under Experimental and Clinical Research, 17, 127–131.
Sanchez-Venegas, J. R., Dinamarca, J., Moraga, A. G., & Gidekel, M. (2009). Molecular characterization of a cDNA encoding Cu/Zn superoxide dismutase from Deschampsia antarctica and its expression regulated by cold and UV stresses. BMC Research Notes, 2, 198.
Kumar, A., Dutt, S., Bagler, G., Ahuja, P. S., & Kumar, S. (2012). Engineering a thermo-stable superoxide dismutase functional at sub-zero to >50 degrees C, which also tolerates autoclaving. Scientific Reports, 2, 387.
Garcia-Echauri, S. A., Gidekel, M., Moraga, A. G., Ordonez, L. G., Contreras, J. A. R., Barba de la Rosa, A. P., & De Leon Rodriguez, A. (2009). Heterologous expression of a novel psychrophilic Cu/Zn superoxide dismutase from Deschampsia antarctica. Process Biochemistry, 44, 969–974.
Bradford, M. M. (1976). A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Analytical Biochemistry, 72, 248–254.
Beauchamp, C., & Fridovich, I. (1971). Superoxide dismutase: improved assays and an assay applicable to acrylamide gels. Analytical Biochemistry, 44, 276–287.
McCord, J. M., & Fridovich, I. (1969). Superoxide dismutase. An enzymic function for erythrocuprein (hemocuprein). Journal of Biological Chemistry, 244, 6049–6055.
Yogavel, M., Gill, J., Mishra, P. C., & Sharma, A. (2007). SAD phasing of a structure based on cocrystallized iodides using an in-house CuK alpha X-ray source: effects of data redundancy and completeness on structure solution. Acta Crystallography D, 63, 931–934.
Yogavel, M., Mishra, P. C., Gill, J., Bhardwaj, P. K., Dutt, S., Kumar, S., Ahuja, P. S., & Sharma, A. (2008). Structure of a superoxide dismutase and implications for copper-ion chelation. Acta crystallographica. Section D, Biological crystallography, D64, 892–901.
Chodanowski, P., Grosdidier, A., Feytmans, E., Michielin, O. (2008) Local alignment refinement using structural assessment. Plos One 3.
Perry, J. J. P., Shin, D. S., Getzoff, E. D., & Tainer, J. A. (2010). The structural biochemistry of the superoxide dismutases. Bba-Proteins Proteomics, 1804, 245–262.
Wang, S. Y., Shao, B., Liu, S. T., Ye, X. Y., & Rao, P. F. (2012). Purification and characterization of Cu, Zn-superoxide dismutase from black soybean. Food Research International, 47, 374–379.
Kumar, S., Dhillon, S., Singh, D., & Singh, R. (2004). Partial purification and characterization of superoxide dismutase from tomato (Lycopersicon esculentum) fruit. Journal of Food Sciences and Nutrition, 9, 283–288.
Zheng, Z., Jiang, Y. H., Miao, J. L., Wang, Q. F., Zhang, B. T., & Li, G. Y. (2006). Purification and characterization of a cold-active iron superoxide dismutase from a psychrophilic bacterium, Marinomonas sp NJ522. Biotechnology Letters, 28, 85–88.
Sahoo, R., Kumar, S., & Ahuja, P. S. (2001). Induction of a new isozyme of superoxide dismutase at low temperature in Potentilla astrisanguinea Lodd. variety argyrophylla (Wall. ex. Lehm) Griers. Journal of Plant Physiology, 158, 1093–1097.
Giannopolitis, C. N., & Ries, S. K. (1977). Superoxide dismutases. I: occurrence in higher plants. Plant Physiology, 59, 309–314.
Asada, K., Takahashi, M., & Nagate, M. (1974). Assay and inhibitors of spinach superoxide dismutase. Agricultural and Biological Chemistry, 38, 471–473.
Acknowledgments
This work was partially financed by CONACyT-Básicas Grant No. 178988. Juan Rojas thanks CONACyT for his scholarship No. 204213. The authors thank Leandro G. Ordoñez for technical support and Jennifer Ecklerly for English correction. We thank the Chilean Antarctic Institute (INACH) for the logistic support during the stay in the Scientific Base “Prof. Julio Escudero,” King George Island, Antarctic.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rojas-Contreras, J.A., de la Rosa, A.P.B. & De León-Rodríguez, A. Expression and Characterization of a Recombinant Psychrophilic Cu/Zn Superoxide Dismutase from Deschampsia antarctica E. Desv. [Poaceae]. Appl Biochem Biotechnol 175, 3287–3296 (2015). https://doi.org/10.1007/s12010-015-1496-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-015-1496-3