Abstract
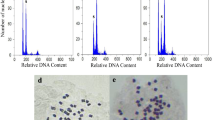
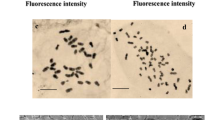
This study is aimed at testing the efficiency of colchicine on inducing polyploidy in Cannabis sativa L. and investigation of effects of polyploidy induction on some primary and secondary metabolites. Shoot tips were treated with three different concentrations of colchicine (0, 0.1, 0.2 % w/v) for 24 or 48 h. The biggest proportion of the almost coplanar tetraploids (43.33 %) and mixoploids (13.33 %) was obtained from the 24-h treatment in 0.2 and 0.1 % w/v, respectively. Colchicine with 0.2 % concentration and 48 h duration was more destructive than 24 h. The ploidy levels were screened with flow cytometry. The biochemical analyses showed that reducing sugars, soluble sugars, total protein, and total flavonoids increased significantly in mixoploid plants compared with tetraploid and diploid plants. Tetraploid plants had a higher amount of total proteins, total flavonoids, and starch in comparison with control plants. The results showed that polyploidization could increase the contents of tetrahydrocannabinol in mixoploid plants only, but tetraploid plants had lower amounts of this substance in comparison with diploids. Also, we found such changes in protein concentration in electrophoresis analysis. In overall, our study suggests that tetraploidization could not be useful to produce tetrahydrocannabinol for commercial use, and in this case, mixoploids are more suitable.


Similar content being viewed by others
References
Vogl, C. (2004), JIH. 9, 37–49
Williamson, E. M., & Evans, F. J. (2000). Drugs, 60, 1303–1314.
Hammond, C. T., & Mahlberg, P. G. (1977). American Journal of Botany, 64, 1023–1031.
Alexander, A., Smith, P. F., & Rosengren, R. J. (2009). Cancer Letters, 285, 6–12.
Truta, E., Gille, E., Toth, E., & Maniu, M. (2002). Journal of Applied Genetics, 43, 451–462.
Griesbach, R. J., & Bhat, R. N. (1990). Horticultural Science, 25, 1284–1286.
Chakraborti, S. P., Vijayan, K., Roy, B. N., & Qadri, S. M. (1998). Plant Cell Reports, 17, 799–803.
Nakano, M. T., Nomizu, K., Mizunashi, M., Suzuki, S., Mori, S., Kuwayama, M., et al. (2006). Horticultural Science, 110, 366–371.
Stanys, V., Weckman, A., Staniene, G., & Duchovskis, P. (2006). Plant Cell Tissue and Organ, 84, 263–268.
GU, X. F., Yang, A. F., Meng, H., & Zhang, J. R. (2005). Plant Cell Reports, 24, 671–676.
Lichtenthaler, H. K. (1987). Methods in Enzymology, 148, 350–382.
Roe, J. H. (1955). The Journal of Biological Chemistry, 212, 335–343.
Somogy, M. (1952). The Journal of Biological Chemistry, 195, 19–29.
Thayumanavan, B., & Sadasivam, S. (1984). Nutrition, 34, 253.
Updegroff, D. M. (1969). Method Biochemistry Analytical, 32, 420.
Wagner, G. J. (1970). Plant Physiology, 64, 88–93.
Krizek, D. T., Britz, S. J., & Mirecki, R. M. (1998). Planta, 103, 1–7.
Bradford, M. M. (1976). Analytical Biochemistry, 72, 248–254.
Rustichelli, C., Ferioli, V., Baraldi, M., Zanoli, P., & Gamberini, G. (1998). Chromatographia, 47, 215–222.
Parry, M. A. J., Schmidt, C. N. G., Cornelium, M. J., Millard, B. N., Burton, S., et al. (1987). Journal of Experimental Botany, 38, 1260–1271.
Xu, L., Najeeb, U., Naeem, M. S., Daud, M. K., Cao, J. S., Gong, H. J., Shen, W. Q., & Zhou, W. J. (2010). Planta, 54, 659–663.
Phulari, S. S. (2011). Botany, 1, 207–210.
Jaskani, M. J., Kwon, S. W., & Kim, D. H. (2005). Euphytica, 145, 259–268.
Grange, S., Leskovar, D. I., Pike, L. M., & Cobb, B. G. (2003). Journal of the American Society for Horticultural Science, 128, 253–259.
Winkel-Shirley, B. (2001). Plant Physiology, 126, 485–493.
Buchert, J., Koponen, J. M., Suutarinen, M., Mustranta, A., Lille, M., Torronen, R., & Poutanen, K. (2005). Journal of the Science of Food and Agriculture, 85, 2548–2556.
Knekt, P., Kumpulainen, J., Järvinen, R., Rissanen, H., Heliövaara, M., Reunanen, A., Hakulinen, T., & Aromaa, A. (2002). The American Journal of Clinical Nutrition, 76, 560–568.
Bretagnolle, F., & Tansley, T. J. D. (1995). New Phytologist, 129, 1–22.
Eichler, M., Spinedi, L., Unfer-Grauwiler, S., Bodmer, M., Surber, C., Luedi, M., & Drewe, J. (2012). Journal of Medicine Plant Natural Products Research, 78, 686–691.
Dhawan, O. P., & Lavania, U. C. (1996). Euphytica, 87, 81–89.
Acknowledgments
The authors would like to thank Mr. Pooya Bagheri (Alberta University, Edmonton) for his help to improve the manuscript level.
Author information
Authors and Affiliations
Corresponding author
Additional information
Key Message
All of the analyzed biochemical parameters except photosynthetic parameters showed significant difference under polyploidy condition. These changes were not suitable for Cannabis sativa as a medicinal plant.
Rights and permissions
About this article
Cite this article
Bagheri, M., Mansouri, H. Effect of Induced Polyploidy on Some Biochemical Parameters in Cannabis sativa L.. Appl Biochem Biotechnol 175, 2366–2375 (2015). https://doi.org/10.1007/s12010-014-1435-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-014-1435-8