Abstract
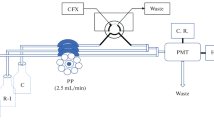
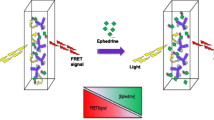
Pepsin (Pep) accelerated the electron transferring rate of excited 3-aminophathlate and enhanced luminol-dissolved oxygen chemiluminescence (CL) intensity, and the flow injection (FI) luminol-Pep CL system was first developed. It was found that the CL intensity of luminol-Pep reaction could be remarkably inhibited by pseudoephedrine (PE); the decrement of CL intensity was linear to the logarithm of PE concentration in the range of 0.1∼100.0 nmol L−1 with a detection limit of 0.03 nmol mL−1 (3σ). At a flow rate of 2.0 mL min−1, the complete process including washing and sampling was performed within 40 s, offering a sample throughput of 90 h−1. This proposed method was successfully applied to determining PE in rat serum for 18 h after intragastric administration with the elimination ratio of 42.34 % and recoveries from 90.3 to 110.6 %. The pharmacokinetic results showed that PE could be rapidly absorbed into serum with peak concentration (C max) of 1.45 ± 0.18 g L−1 at the time (T max) of 1.49 ± 0.02 h; the absorption half-life (0.35 ± 0.04 h), elimination half-life (1.86 ± 0.24 h), the area under curve (109.81 ± 6.03 mg L−1 h−1), mean residence time (3.82 ± 0.27 h), and elimination rate constant (2.26 ± 0.23 L g−1 h−1) in rats vivo were derived, respectively. The possible CL mechanism of luminol-Pep-PE reaction was discussed by FI-CL, fluorescence, and molecular docking (MD) methods.

ᅟ






Similar content being viewed by others
References
Renaud, J. P., & Delsuc, M. A. (2009). Current Opinion in Pharmacology, 9, 622–628.
Azevedo de, W.F.J. Caceres, Pauli, R.A. Timmers, I. L.F. Barcellos, G.B. Rocha, K.B. & Soares, M.B. (2009). Current Drug Targets, 10, 271–278.
Huang, S. M., Zhao, H., Lee, J. I., Reynolds, K., Zhang, L., Temple, R., & Lesko, L. J. (2010). Clinical Pharmacology and Therapeutics, 87, 497–503.
Minai, R., Matsuo, Y., Onuki, H., & Hirota, H. (2008). Proteins, 72, 367–381.
Colinge, J., Rix, U., Bennett, K. L., & Superti-Furga, G. (2012). Journal of Proteomics, 6, 102–116.
Yang, R., Li, S. Q., & Zhang, Q. H. (2004). Journal of Agricultural and Food Chemistry, 52, 7400–7406.
Dunn, B. M. (2002). Chemistry Review, 102, 4431–4458.
Antonov, V. K., Ginodman, L. M., Kapitannikov, Y. V., Barshevskaya, T. N., Gurova, A. G., & Rumsh, L. D. (1978). FEBS Letters, 88, 87–90.
Dee, D. R., & Yada, R. Y. (2009). Biochemistry, 49, 365–371.
Boeris, V., Micheletto, Y., Lionzo, M., Silveira, N. P. D., & Picó, G. (2011). Carbohydrate Polymers, 84, 459–464.
Zeng, H. J., Liang, H. L., You, J., & Qu, L. B. (2013). luminescence. doi:10.1002/bio.2610.
Huang, Y. B., Yan, J., Liu, B. Z., Yu, Z., Gao, X. Y., Tang, Y. C., & Zi, Y. Q. (2010). Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy, 75, 1024–1029.
Zhang, H. M., Cao, J., Fei, Z. H., & Wang, Y. Q. (2012). Journal of Molecular Structure, 1021, 34–39.
Lian, S., Wang, G., Zhou, L., & Yang, D. (2013). Luminescence, 28, 967–972.
Wang, Y. Q., & Zhang, H. M. (2013). Journal of Agricultural and Food Chemistry, 61, 11191–11200.
Hwang, S. S., Gorsline, J., Louie, J., Dye, D., Guinta, D., & Hamel, L. (1995). The Journal of Clinical Pharmacology, 35, 259.
Lloyd, A., Russell, M., Blanes, L., Doble, P., & Roux, C. (2013). Forensic Science International, 228, 8–14.
Barroso, O., Goudreault, D. C., Banús, M. L., Ayotte, C., Mazzoni, I., Boghosian, T., & Rabin, O. (2012). Drug Testing and Analysis, 4, 320–329.
Louhaichi, M. R., Jebali, S., Loueslati, M. H., Adhoum, N., & Monser, L. (2009). Talanta, 78, 991–997.
Liu, Y. M., Tian, W., Jia, Y. X., & Yue, H. Y. (2009). Biomedical Chromatography, 23, 1138–1144.
Deng, D. L., Deng, H., Zhang, L. C., & Su, Y. Y. (2014). Journal of Chromatographic Science, 52, 357–362.
Lee, M. J., Lee, H. W., Kang, J. M., Seo, J. H., Tak, S. K., Shim, W., Yim, S. V., Hong, S. J., & Lee, K. T. (2010). Biomedical Chromatography, 24, 1031–1037.
Li, H., Zhang, C., Wang, J., Jiang, Y., Fawcett, J. P., & Gu, J. (2010). Journal of Pharmaceutical and Biomedical Analysis, 51, 716–722.
Ryu, J. K., & Yoo, S. D. (2012). Journal of Pharmacy and Pharmaceutical Sciences, 15, 519–527.
Das, S., Powe, A. M., Baker, G. A., Valle, B., EI-Zahab, B., Sintim, H. O., Lowry, M., Fakayode, S. O., Mcarroll, M. E., Patonay, G., Li, M., Strongin, R. M., Geng, M. L., & Warner, I. M. (2012). Analytical Chemistry, 84, 597–625.
Rodriguez-Orozco, A. R., Ruiz-Reyes, H., & Medina-Serriteno, N. (2010). Mini Reviews in Medicinal Chemistry, 10, 1393–1400.
Tsogas, G. Z., Giokas, D. L., & Vlessidis, A. G. (2014). Analytical Chemistry, 86, 3484–3492.
Tan, X. J., Wang, Z. M., Chen, D. H., Luo, K., Xiong, X. Y., & Song, Z. H. (2014). Chemosphere, 108, 26–32.
J.R. Lakowicz (1999) 2nd ed. Plenum Press, New York, pp 27–60.
Lakowicz, J. R., & Weber, G. (1973). Biochemistry, 12, 4161–4170.
He, X. L., Xie, X. F., Shao, X. D., & Song, Z. H. (2010). Luminescence, 25, 384–388.
Wang, Z. M., & Song, Z. H. (2010). The Analyst, 135, 2546–2553.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (no. 21275118) and the Open Fund from Key Laboratory of Synthetic and Natural Functional Molecule Chemistry of Ministry of Education, China.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Luo, K., Li, Y., Zheng, X. et al. Pharmacokinetic of Pseudoephedrine in Rat Serum with Luminol-Pepsin Chemiluminescence System by Flow Injection Analysis. Appl Biochem Biotechnol 175, 1805–1816 (2015). https://doi.org/10.1007/s12010-014-1396-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-014-1396-y