Abstract
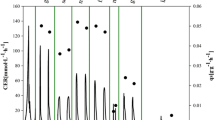
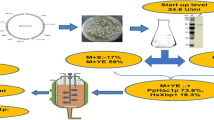
In this work, a combined strategy was developed to improve the production of glucose oxidase (GOD) (EC 1.1.3.4) in Pichia pastoris. One of the main challenges facing protein production by the high-density fermentation of P. pastoris is the high demand for oxygen. Another challenge is how to balance a reduction in oxygen consumption and its effects on protein production. Herein, a combined strategy involving mannitol co-feeding, two-stage methanol induction, and the co-expression of the transcriptional activator general control non-derepressible 4 (GCN4) from P. pastoris was used. A two-stage, co-feeding strategy, based on a mannitol/methanol mixture in a 3-L fermentor was used to enhance cell viability and protein production. This resulted in an increased GOD yield of 1208.2 U/mL compared with a control strain (427.6 U/mL). An increase in the copy number of the GCN4 gene enhanced the GOD yield (1634.7 U/mL) by 2.8-fold and the protein concentration (19.55 g/L) by 1.58-fold compared with the control (7.59 g/L). This strategy illustrates a way to overcome the high oxygen requirement during high-density fermentation of P. pastoris and balances the reduction of oxygen consumption and protein production. Moreover, the series of strategies presented in this work provide valuable and novel information for the industrial production of GOD.








Similar content being viewed by others
References
Bankar, S. B., Bule, M. V., Singhal, R. S., & Ananthanarayan, L. (2009). Glucose oxidase–an overview. Biotechnology Advances, 27, 489–501.
Bankar, S. B., Bule, M. V., Singhal, R. S., & Ananthanarayan, L. (2009). Optimization of Aspergillus niger fermentation for the production of glucose oxidase. Food and Bioprocess Technology, 2, 344–352.
Çalık, P., Bozkurt, B., Zerze, G. H., İnankur, B., Bayraktar, E., Boy, E., Orman, M. A., Açık, E., & Özdamar, T. H. (2013). Effect of co-substrate sorbitol different feeding strategies on human growth hormone production by recombinant Pichia pastoris. Journal Chemical Technology and Biotechnology, 88, 1631–1640.
Çelik, E., Çalık, P., & Oliver, S. G. (2010). Metabolic flux analysis for recombinant protein production by Pichia pastoris using dual carbon sources: effects of methanol feeding rate. Biotechnology and Bioengineering, 105, 317–329.
Cereghino, J. L., & Cregg, J. M. (2000). Heterologous protein expression in the methylotrophic yeast Pichia pastoris. FEMS Microbiology Review, 24, 45–66.
Cos, O., Ramón, R., Montesinos, J., & Valero, F. (2006). Operational strategies, monitoring and control of heterologous protein production in the methylotrophic yeast Pichia pastoris under different promoters: a review. Microbial Cell Factories, 5, 17–38.
Cos, O., Serrano, A., Montesinos, J. L., Ferrer, P., Cregg, J. M., & Valero, F. (2005). Combined effect of the methanol utilization (Mut) phenotype and gene dosage on recombinant protein production in Pichia pastoris fed-batch cultures. Journal of Biotechnology, 116, 321–335.
Crognale, S., Pulci, V., Brozzoli, V., Petruccioli, M., & Federici, F. (2006). Expression of Penicillium variabile P16 glucose oxidase gene in Pichia pastoris and characterization of the recombinant enzyme. Enzyme and Microbial Technology, 39, 1230–1235.
Damasceno, L. M., Huang, C.-J., & Batt, C. A. (2012). Protein secretion in Pichia pastoris and advances in protein production. Applied Microbiology and Biotechnology, 93, 31–39.
Fiedurek, J., & Gromada, A. (2000). Production of catalase and glucose oxidase by Aspergillus niger using unconventional oxygenation of culture. Journal of Applied Microbiology, 89, 85–89.
Frederick, K., Tung, J., Emerick, R., Masiarz, F., Chamberlain, S., Vasavada, A., Rosenberg, S., Chakraborty, S., Schopfer, L., & Schopter, L. (1990). Glucose oxidase from Aspergillus niger. Cloning, gene sequence, secretion from Saccharomyces cerevisiae and kinetic analysis of a yeast-derived enzyme. Journal of Biological Chemistry, 265, 3793–3802.
Gasser, B., Saloheimo, M., Rinas, U., Dragosits, M., Rodríguez-Carmona, E., Baumann, K., Giuliani, M., Parrilli, E., Branduardi, P., & Lang, C. (2008). Protein folding and conformational stress in microbial cells producing recombinant proteins: a host comparative overview. Microbial Cell Factories, 7, 11.
Gerritsen, M., Kros, A., Lutterman, J., Nolte, R., & Jansen, J. (2001). A percutaneous device as model to study the in vivo performance of implantable amperometric glucose sensors. Journal of Materials Science. Material in Medicine, 12, 129–134.
Guo, Y., Lu, F., Zhao, H., Tang, Y., & Lu, Z. (2010). Cloning and heterologous expression of glucose oxidase gene from Aspergillus niger Z-25 in Pichia pastoris. Applied Biochemistry and Biotechnology, 162, 498–509.
Harding, H., Zhang, Y., Zeng, H., Novoa, I., Lu, P., Calfon, M., Sadri, N., Yun, C., Popko, B., & Paules, R. (2003). An integrated stress response regulates amino acid metabolism and resistance to oxidative stress. Molecular Cell, 11, 619.
Hatzinikolaou, D., & Macris, B. (1995). Factors regulating production of glucose oxidase by Aspergillus niger. Enzyme and Microbial Technology, 17, 530–534.
Haynes, C. M., Titus, E. A., & Cooper, A. A. (2004). Degradation of misfolded proteins prevents ER-derived oxidative stress and cell death. Molecular Cell, 15, 767–776.
Hohenblum, H., Gasser, B., Maurer, M., Borth, N., & Mattanovich, D. (2004). Effects of gene dosage, promoters, and substrates on unfolded protein stress of recombinant Pichia pastoris. Biotechnology and Bioengineering, 85, 367–375.
Inan, M., & Meagher, M. M. (2001). Non-repressing carbon sources for alcohol oxidase (AOX1) promoter of Pichia pastoris. Journal of Bioscience and Bioengineering, 92, 585–589.
Jiménez-Martí, E., Zuzuarregui, A., Gomar-Alba, M., Gutiérrez, D., Gil, C., & Del Olmo, M. (2011). Molecular response of Saccharomyces cerevisiae wine and laboratory strains to high sugar stress conditions. International Journal of Food Microbiology, 145, 211–220.
Jordà, J., Jouhten, P., Cámara, E., Maaheimo, H., Albiol, J., & Ferrer, P. (2012). Metabolic flux profiling of recombinant protein secreting Pichia pastoris growing on glucose: methanol mixtures. Microbial Cell Factories, 11, 57.
Jungo, C., Rérat, C., Marison, I. W., & von Stockar, U. (2006). Quantitative characterization of the regulation of the synthesis of alcohol oxidase and of the expression of recombinant avidin in a Pichia pastoris Mut+ strain. Enzyme and Microbial Technology, 39, 936–944.
Jungo, C., Schenk, J., Pasquier, M., Marison, I. W., & von Stockar, U. (2007). A quantitative analysis of the benefits of mixed feeds of sorbitol and methanol for the production of recombinant avidin with Pichia pastoris. Journal of Biotechnology, 131, 57–66.
Kapat, A., Jung, J.-K., & Park, Y.-H. (1998). Improvement of extracellular recombinant glucose oxidase production in fed-batch culture of Saccharomyces cerevisiae: effect of different feeding strategies. Biotechnological Letters, 20, 319–323.
Kapat, A., Jung, J. K., & Park, Y. H. (2001). Enhancement of glucose oxidase production in batch cultivation of recombinant Saccharomyces cerevisiae: optimization of oxygen transfer condition. Journal of Applied Microbiology, 90, 216–222.
Krainer, F. W., Dietzsch, C., Hajek, T., Herwig, C., Spadiut, O., & Glieder, A. (2012). Recombinant protein expression in Pichia pastoris strains with an engineered methanol utilization pathway. Microbial Cell Factories, 11, 22–35.
Kruger, N. J. (1994), The Bradford method for protein quantitation. In J. M. Walker (Ed.), Basic protein and peptide protocols (pp. 9–15). New York: Springer.
Lu, T., Peng, X., Yang, H., & Ji, L. (1996). The production of glucose oxidase using the waste myceliums of Aspergillus niger and the effects of metal ions on the activity of glucose oxidase. Enzyme and Microbial Technology, 19, 339–342.
Malherbe, D., Du Toit, M., Otero, R. C., Van Rensburg, P., & Pretorius, I. (2003). Expression of the Aspergillus niger glucose oxidase gene in Saccharomyces cerevisiae and its potential applications in wine production. Applied Microbiology and Biotechnology, 61, 502–511.
Niu, H., Jost, L., Pirlot, N., Sassi, H., Daukandt, M., Rodriguez, C., & Fickers, P. (2013). A quantitative study of methanol/sorbitol co-feeding process of a Pichia pastoris Mut+/pAOX1-lacZ strain. Microbial Cell Factories, 12, 33.
Patil, C. K., Li, H., & Walter, P. (2004). Gcn4p and novel upstream activating sequences regulate targets of the unfolded protein response. PLoS Biology, 2, 1208–1223.
Paulová, L., Hyka, P., Branská, B., Melzoch, K., & Kovar, K. (2012). Use of a mixture of glucose and methanol as substrates for the production of recombinant trypsinogen in continuous cultures with Pichia pastoris Mut+. Journal of Biotechnology, 157, 180–188.
Petruccioli, M., Federici, F., Bucke, C., & Keshavarz, T. (1999). Enhancement of glucose oxidase production by Penicillium variabile P16. Enzyme and Microbial Technology, 24, 397–401.
Petruccioli, M., Piccioni, P., Federici, F., & Polsinelli, M. (1995). Glucose oxidase overproducing mutants of Penicillium variabile (P16). FEMS Microbiology Letters, 128, 107–111.
Pluschkell, S., Hellmuth, K., & Rinas, U. (1996). Kinetics of glucose oxidase excretion by recombinant Aspergillus niger. Biotechnology and Bioengineering, 51, 215–220.
Ramón, R., Ferrer, P., & Valero, F. (2007). Sorbitol co-feeding reduces metabolic burden caused by the overexpression of a Rhizopus oryzae lipase in Pichia pastoris. Journal of Biotechnology, 130, 39–46.
Rando, D., Kohring, G.-W., & Giffhorn, F. (1997). Production, purification and characterization of glucose oxidase from a newly isolated strain of Penicillium pinophilum. Applied Microbiology and Biotechnology, 48, 34–40.
Rocha, S. N., Abrahão-Neto, J., Cerdán, M. E., González-Siso, M. I. and Gombert, A. K. (2010). Heterologous expression of glucose oxidase in the yeast Kluyveromyces marxianus. Microbial Cell Factories, 9:4. doi: 10.1186/1475-2859-9-4
Rutkowski, D. T., & Kaufman, R. J. (2004). A trip to the ER: coping with stress. Trends in Cell Biology, 14, 20–28.
Sato, Y., & Inaba, K. (2012). Disulfide bond formation network in the three biological kingdoms, bacteria, fungi and mammals. FEBS Journal, 279, 2262–2271.
Sha, C., Yu, X.-W., Li, F., & Xu, Y. (2013). Impact of gene dosage on the production of lipase from Rhizopus chinensis CCTCC M201021 in Pichia pastoris. Applied Biochemistry and Biotechnology, 169, 1160–1172.
Taboada-Puig, R., Junghanns, C., Demarche, P., Moreira, M., Feijoo, G., Lema, J., & Agathos, S. (2011). Combined cross-linked enzyme aggregates from versatile peroxidase and glucose oxidase: Production, partial characterization and application for the elimination of endocrine disruptors. Bioresource Technology, 102, 6593–6599.
Tu, B. P., & Weissman, J. S. (2004). Oxidative protein folding in eukaryotes mechanisms and consequences. Journal of Cell Biology, 164, 341–346.
Wang, Z., Wang, Y., Zhang, D., Li, J., Hua, Z., Du, G., & Chen, J. (2010). Enhancement of cell viability and alkaline polygalacturonate lyase production by sorbitol co-feeding with methanol in Pichia pastoris fermentation. Bioresource Technology, 101, 1318–1323.
Xiao, A., Zhou, X., Zhou, L., & Zhang, Y. (2006). Improvement of cell viability and hirudin production by ascorbic acid in Pichia pastoris fermentation. Applied Microbiology and Biotechnology, 72, 837–844.
Xie, J., Zhou, Q., Du, P., Gan, R., & Ye, Q. (2005). Use of different carbon sources in cultivation of recombinant Pichia pastoris for angiostatin production. Enzyme and Microbial Technology, 36, 210–216.
Yamaguchi, M., Tahara, Y., Nakano, A., & Taniyama, T. (2007). Secretory and continuous expression of Aspergillus niger glucose oxidase gene in Pichia pastoris. Protein Expression and Purification, 55, 273–278.
Acknowledgments
This project was financially supported by the National High Technology Research and Development Program of China (863 Program, 2011AA100905), the Major State Basic Research Development Program of China (973 Program, 2013CB733902), Synergetic Innovation Center Of Food Safety and Nutrition, the Natural Science Foundation of Jiangsu Province (BK2012553), the China Postdoctoral Science Foundation(2013M540538), and the 111 Project (111-2-06).
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Gu, L., Zhang, J., Liu, B. et al. High-Level Extracellular Production of Glucose Oxidase by Recombinant Pichia Pastoris Using a Combined Strategy. Appl Biochem Biotechnol 175, 1429–1447 (2015). https://doi.org/10.1007/s12010-014-1387-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-014-1387-z