Abstract
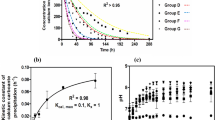
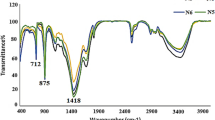
Contamination by radioactive strontium (90Sr) is a significant environmental problem. Ureolytically driven calcium carbonate precipitation has been proposed for use in geotechnical engineering for soil remediation applications. In this study, 68 ureolytic bacterial strains were newly isolated from various environments. Of these, 19 strains were selected based on ureolytic activity shown when cultured on urea agar plates and identified through 16S rRNA gene sequencing. From these selected strains, Sporosarcina pasteurii WJ-2 (WJ-2) was selected for subsequent study. A simple method was developed to determine the effectiveness of microbially induced calcite precipitation (MICP). Unlike any other methods, it does not require advanced skills and sophisticated tools. Using this method, we were able to determine the ability of the bioconsolidated sand to retard the flow of crystal violet through the 25-mL column. Also, MICP by WJ-2 was evaluated for its potential to counteract Sr contamination in column experiments using natural sand. WJ-2-induced precipitation led to successful sequestration of approximately 80 % of the Sr from the soluble fraction of the sand. The utility of MICP in bioremediation was further confirmed through X-ray diffraction, scanning electron microscopy, and inductively coupled plasma mass spectrometry.







Similar content being viewed by others
References
Warren, L. A., Maurice, P. A., Parmar, N., & Ferris, F. G. (2001). Geomicrobiology Journal, 18, 93–115.
Fujita, Y., Redden, G. D., Ingram, J. C., Cortez, M. M., Ferris, G. F., & Smith, R. W. (2004). Geochimica et Cosmochimica Acta, 68, 3261–3270.
Davis, J. A., Fuller, C. C., & Cook, A. D. (1987). Geochimica et Cosmochimica Acta, 51, 1477–1490.
Tesoriero, A. J., & Pankow, J. F. (1996). Geochimica et Cosmochimica Acta, 60, 1053–1063.
Curti, E. (1999). Applied Geochemistry, 14, 433–445.
Varenyam, A. X., Pan, L., Zhang, D. Y., & Fu, Q. L. (2011). Journal of Microbiology and Biotechnology, 22, 244–247.
Boquet, E., Boronat, A., & Cormenzana, A. R. (1973). Nature, 246, 527–529.
Rivadeneyra, M. A., Delgado, R., Moral, A. D., Ferrer, M. R., & Cormenzana, A. R. (1993). FEMS Microbiology Ecology, 13, 197–204.
Whiffin, V. S., Paassen, L. A., & Harkes, M. P. (2007). Geomicrobiology Journal, 24, 417–423.
Hammes, F., & Verstraete, W. (2002). Reviews in Environmental Science and Biotechnology, 1, 3–7.
Stocks-Fischer, S., Galinat, J. K., & Bang, S. S. (1999). Soil Biology & Biochemistry, 31, 1563–1571.
Tamura, K., Peterson, D., Peterson, N., Stecher, G., Nei, M., & Kumar, S. (2011). Molecular Biology and Evolution, 28, 2731–2739.
Weatherburn, M. W. (1967). Analytical Chemistry, 39, 971–974.
Bradford, M. M. (1976). Analytical Biochemistry, 72, 248–254.
McCarty, G. W., Shogren, D. R., & Bremner, J. M. (1992). Biology and Fertility of Soils, 12, 261–264.
Paulson, K. N., & Kurtz, L. T. (1969). Soil Science Society of America Journal, 33, 897–901.
Chunxiang, Q., Jianyun, W., Ruixing, W., & Liang, C. (2009). Materials Science and Engineering: C, 29, 1273–1280.
McConnaughey, T. A., & Whelan, J. F. (1997). Earth-Science Reviews, 42, 95–117.
Bachmeier, K. L., Williams, A. E., Warmington, J. R., & Bang, S. S. (2002). Journal of Biotechnology, 93, 171–181.
DeJong, J. T., Mortensen, B. M., Martinez, B. C., & Nelson, D. C. (2010). Ecological Engineering, 36, 197–210.
Whiffin, V. S., van Paassen, L. A., & Harkes, M. P. (2007). Geomicrobiology Journal, 24, 417–423.
Warren, L. A., Maurice, P. A., Parmar, N., & Ferris, F. G. (2001). Geomicrobiology Journal, 18, 93–115.
Acknowledgments
This research was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF), funded by the Ministry of Education (NRF-2011-0025229).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kang, CH., Choi, JH., Noh, J. et al. Microbially Induced Calcite Precipitation-based Sequestration of Strontium by Sporosarcina pasteurii WJ-2. Appl Biochem Biotechnol 174, 2482–2491 (2014). https://doi.org/10.1007/s12010-014-1196-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-014-1196-4