Abstract
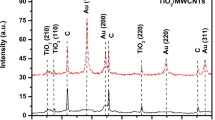
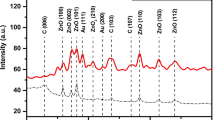
Highly sensitive potassium (K)-doped carbon nanotube (CNT) and polypyrrole (PPy) nanocomposite membrane-based enzyme field effect transistor (ENFET) has been fabricated on indium tin oxide (ITO) for detection of cholesterol. P-type graphene has been deposited as substrate on ITO glass electrochemically. N-type graphene has been deposited in source and drain regions. Zirconium dioxide (ZrO2) has been deposited on the channel region as gate insulator. K/PPy/CNT composite has been deposited as sensing membrane on the top of ZrO2 layer; 1 μl of cholesterol oxidase (ChOx) has been immobilized on K/PPy/CNT membrane via physical adsorption technique. The response of K/PPy/CNT/FET has been studied using Agilent 3458A digital multimeter in presence of phosphate buffer saline (PBS) of 50 mM, pH 7.0 and 0.9 % NaCl contained in a glass pot. During measurement, 20 μl cholesterol solutions (0.5 to 25 mM) were poured into the pot through micropipette each time. It has been found that K/PPy/CNT/FET has linearly varied from 0.5 to 20 mM. The sensitivity of this FET has been found to be ~400 μA/mM/mm2 with regression coefficient (r) ~ 0.998. The proposed ENFET has response time of 1 s and stability up to 6 months. The experiment has been repeated 10 times, and only 2.0 % output variation has been observed. The limit of detection (LoD) and Michaelis-Menten constant (K m) were found to be ~1.4 and 2.5 mM, respectively. The results obtained in this work show negligible interference (3.7 %) with uric acid, glucose and urea.







Similar content being viewed by others
References
Baynes, J. W., & Dominiczak, M. (2005). Medical biochemistry (2nd ed.). Mosby Ltd: Elsevier.
White, A., Handler, P., Smith, E. L., Hill, R. L., & Lehman, I. R. (1987). Principles of biochemistry (6th ed.). New York: McGraw-Hill Book.
Ahmed, R., Tripathy, N., & Hanh, Y. B. (2013). High-performance cholesterol sensor based on the solution-gated field effect transistor fabricated with ZnO nanorods. Biosensors and Bioelectronics, 45, 181–186.
Arya, S. K., Dutta, M., & Malhotra, B. D. (2008). Recent advances in cholesterol biosensor. Bioelectronics & Biosensors, 23(7), 1083–1100.
Nauck, M., Graziani, M. S., Bruton, D., et al. (1997). Multicenter evaluation of a homogeneous assay for HDL-cholesterol without sample pretreatment. Clinical Chemistry, 43, 1622–1629.
Umar, A., Rahman, M. M., Vassem, M., & Hahn, Y. B. (2009). Ultra-sensitive cholesterol biosensor based on low-temperature grown ZnO nanoparticles. Electrochemistry Communications, 11(1), 118–121.
Motonaka, J., & Faultner, L. R. (1993). Determination of cholesterol and cholesterol ester with novel enzyme microsensors. Analytical Chemistry, 65(22), 3258–3261.
Barik, A., Solanki, P. R., Kaushik, A., et al. (2010). Polyaniline–carboxymethyl cellulose nanocomposite for cholesterol detection. Nanoscience and Nanotechnology, 10, 1–10.
Dhand, C., Arya, S. K., Datta, M., et al. (2008). Polyaniline–carbon nanotube composite film for cholesterol biosensor. Analytical Biochemistry, 383, 194–199.
Janata, J., & Josowicz, M. (2002). Conducting polymers in electronic chemical sensors. Nature materials, 2(1), 19–24.
Dutta, J.C. (2012). Ion sensitive field effect transistor for applications in bioelectronic sensors: a research review. IEEE conference publication. doi: 10.1109/NCCISP.2012.6189704 DOI:10.1109/NCCISP.2012.6189704#blank, 185 – 191.
Bergveld, P. (1970). Development of an ion-sensitive solid-state device for neurophysiological measurements. IEEE Transactions on Biomedical Engineering, 17, 70–71.
Bergveld, P. (1972). Development, operation, and application of the ion-sensitive field-effect transistor as a tool for electrophysiology. IEEE Transactions on Biomedical Engineering, 19(5), 342–451.
Matsuo, T., & Wise, K. D. (1974). An integrated field effect electrode for biopotential recording. EEE Transactions on Biomedical Engineering BME, 21, 485–487.
Bergveld, P. (2003). ISFET, theory and practice. Sensors and Actuators B, 88, 1–20.
Yuqing, M., Jianguo, G., & Jianrong, C. (2003). Ion sensitive field effect transducer-based biosensors. Biotechnology Advances, 21, 527–534.
Ishige, Y., Shimoda, M., & Kamahari, M. (2009). Extended-gate FET-based enzyme sensor with ferrocenyl-alkanethiol modified gold sensing electrode. Biosensors and Bioelectronics, 24, 1096–1102.
Sahoo, R., & Mishro, R. R. (2009). Simulations of carbon nanotube field effect transistors. International Journal of Electronic Engineering Research, 1, 117–125.
Javey, A., Kim, H., Brink, M., et al. (2002). High-κ dielectrics for advanced carbon nanotube transistors and logic gates. Nature Materials, 1, 241–246.
Javey, A., Guo, J., Farmer, D. B., et al. (2004). Carbon nanotube field-effect transistors with integrated ohmic contacts and high-K gate dielectrics. Nano Letters, 4, 447–450.
Dong, Z., Wejinya, U. C., & Chalamalasetty, S. N. S. (2012). Development of CNT-ISFET based pH sensing system using atomic force microscopy. Sensors and Actuators, A: Physical, 173, 293–301.
Chin, S. K., Seath, D., Lam, K. T., et al. (2010). Device physics and characteristics of graphene nanoribbon tunneling FETs. IEEE Transaction on Electron Devices., 57, 3144–3152.
Neto, A. H. C., Guinea, F., Peres, N. M. R., et al. (2009). The electronic properties of graphene. Reviews of Modern Physics, 81, 1,109–163.
Stain, M. R., Unluer, D., & Ghosh, A. (2009). Graphene devices. Interconnect and Circuits Challenges and Opportunities. IEEE, 69–72.
Choudhury, M. R., Yoon, Y., Guo, J., et al. (2011). Graphene nanoribbon FETs: technology exploration for performance and reliability. IEEE Transaction on Nanotechnology, 10, 727–736.
Chen, Z., Lin, Y. M., Rooks, M. J., et al. (2007). Graphene nano-ribbon electronics. Physica E, 40, 228–232.
Lee, R. S., Kim, H. J., Fischer, J. E., et al. (1997). Conductivity enhancement in single-walled carbon nanotube bundles doped with K and Br. Nature, 387, 255–257.
Wang, Z., Liu, J., Liang, Q., et al. (2002). Carbon nanotube-modified electrodes for the simultaneous determination of dopamine and ascorbic acid. Analyst, 127, 653–658.
Guo, M., Chen, J., Li, J., et al. (2004). Carbon nanotubes-based amperometric cholesterol biosensor fabricated through layer-by-layer technique. Electroanalysis, 16, 1992–1998.
Raicopol, M., Prună, A., Damian, C., et al. (2013). Functionalized single-walled carbon nanotubes/polypyrrole composites for amperometric glucose biosensors. Nanoscale Research Letters, 316, 1–8.
Mathur, R. B., Pande, S., Singh, B. P., et al. (2008). Electrical and mechanical properties of multi-walled carbon nanotubes reinforced PMMA and PS composites. Polymer Composites. doi:10.1002/pc.20449.
Du, C., & Pan, N. (2006). High power density supercapacitor electrodes of carbon nanotube films by electrophoretic deposition. Nanotechnology, 17, 5314–5318.
Guo, B., Fang, L., Zhang, B., et al. (2011). Graphene doping: a review. Insciences Journal, 1(2), 80–89.
Gregory, S. D., Gabriella, L., Fang, L., et al. (2010). Ex situ vapor phase boron doping of silicon nanowires using BBr3. Nanoscale, 2, 1165–1170.
Hiroshi, S., & Kioshi, I. (2009). Gas flow sputtering: versatile process for the growth of nanopillars, nanoparticles and epitaxial thin films. Journal of Magnetism and Magnetic Materials, 321, 872–875.
Antti, R., & Mikko, R. (2002). Reaction mechanism studies on the zirconium chloride-water atomic layer deposition process. Journal of Materials Chemistry, 12, 1484–1489.
Javey, A., Tu, R., Farmer, D. B., et al. (2005). High performance n-type carbon nanotube field-effect transistors with chemically doped contacts. Nano Letters, 5, 345–348.
Norouzi, P., Faridbod, F., Esfahani, N. E., et al. (2010). Cholesterol biosensor based on MWCNTs-MnO2 nanoparticles using FFT continuous cyclic voltammetry. International Journal of Electrochemical Science, 5, 1008–1017.
Schisterman, E. F., Moysich, K. B., England, L. J., et al. (2003). Estimation of the correlation coefficient using the Bayesian approach and its applications for epidemiologic research. BMC Medical Research Methodology. doi:10.1186/1471-2288-3-5.
George, W. B. (1982). Standard deviation, standard error. American Journal Diseases Children, 136, 937–941.
Ruecha, N., Rangkupan, R., Rodthongkum, N., et al. (2014). Novel paper-based cholesterol biosensor using graphene/polyvinylpyrrolidone/polyaniline nanocomposite. Biosensors and Bioelectronics, 52, 13–19.
Ali, M. A., Srivastava, S., Solanki, P. R., et al. (2012). Nanostructured anatase-titanium dioxide based platform for application to microfluidics cholesterol biosensor. Applied Physics Letters, 101(084105), 1–5.
Acknowledgments
The authors thank the Council of Scientific and Industrial Research, India, for the award of Senior Research Fellowship to A. Barik (File No. 9/1099(0001)/2013-EMR-I). The authors are also thankful to Tezpur University and the University of Science and Technology, Meghalaya, for providing the facilities in the laboratory.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Barik, M.A., Sarma, M.K., Sarkar, C.R. et al. Highly Sensitive Potassium-Doped Polypyrrole/Carbon Nanotube-Based Enzyme Field Effect Transistor (ENFET) for Cholesterol Detection. Appl Biochem Biotechnol 174, 1104–1114 (2014). https://doi.org/10.1007/s12010-014-1029-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-014-1029-5