Abstract
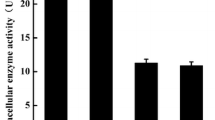
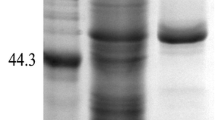
A strain that exhibited intracellular proline-specific aminopeptidase (PAP) activity was isolated from soy sauce koji and identified as Aspergillus oryzae JN-412. The gene coding PAP was cloned and efficiently expressed in Escherichia coli BL21 in a biologically active form. The highest specific activity reached 52.28 U mg−1 at optimum cultivation conditions. The recombinant enzyme was purified 3.3-fold to homogeneity with a recovery of 36.7 % from cell-free extract using Ni-affinity column chromatography. It appeared as a single protein band on SDS-PAGE with molecular mass of approximately 50 kDa. The purified enzyme exhibited the highest activity at 60 °C and pH 7.5. The enzyme activity was inhibited by PMSF and ions like Zn2+ and Cu2+. DTT, β-mercaptoethanol, EDTA, and ions like Co2+, Mg2+, Mn2+, and Ca2+ had no influence on enzyme activity, whereas Ni2+ enhanced the enzyme activity. By using collagen as a substrate, the purified recombinant prolyl aminopeptidase contributed to the hydrolysis of collagen when used in combination with neutral protease, and free amino acids in collagen hydrolysates was significantly increased.




Similar content being viewed by others
References
Taylor, A. (1993). Aminopeptidases: structure and function. FASEB journal : official publication of the Federation of American Societies for Experimental Biology, 7, 290–298.
Rawlings, N. D., & Barrett, A. J. (1993). Evolutionary families of peptidases. Biochemical Journal, 290, 205–218.
Rawlings, N. D., Morton, F. R., & Barrett, A. J. (2006). MEROPS: the peptidase database. Nucleic Acids Research, 34, D270–D272.
Morel, F., Gilbert, C., Geourjon, C., Frot-Coutaz, J., Portalier, R., & Atlan, D. (1999). The prolyl aminopeptidase from Lactobacillus delbrueckii subsp. bulgaricus belongs to the alpha/beta hydrolase fold family. Biochimica Et Biophysica Acta, 1429, 501–505.
Kitazono, A., Ito, K., & Yoshimoto, T. (1994). Prolyl aminopeptidase is not a sulfhydryl enzyme: identification of the active serine residue by site-directed mutagenesis. Journal of Biochemistry, 116, 943–945.
Polgar, L. (2002). The prolyl oligopeptidase family. Cellular and Molecular Life Sciences CMLS, 59, 349–362.
Arima, J., Uesugi, Y., Uraji, M., Iwabuchi, M., & Hatanaka, T. (2006). Dipeptide synthesis by an aminopeptidase from Streptomyces septatus TH-2 and its application to synthesis of biologically active peptides. Applied and Environmental Microbiology, 72, 4225–4231.
Murai, A., Tsujimoto, Y., Matsui, H., & Watanabe, K. (2004). An Aneurinibacillus sp. strain AM-1 produces a proline-specific aminopeptidase useful for collagen degradation. Journal of Applied Microbiology, 96, 810–818.
Nandan, A., Pandey, A., & Nampoothiri, K. M. (2011). Proline-specific extracellular aminopeptidase purified from Streptomyces lavendulae. Applied Biochemistry and Biotechnology, 163, 994–1001.
Bolumar, T., Sanz, Y., Aristoy, M. C., & Toldra, F. (2003). Purification and characterization of a prolyl aminopeptidase from Debaryomyces hansenii. Applied and Environmental Microbiology, 69, 227–232.
Izawa, N., Ishikawa, S., Tanokura, T., Ohta, K., & Hayashi, K. (1997). Purification and characterization of Aeromonas caviae aminopeptidase possessing debittering activity. Journal of Agricultural and Food Chemistry, 45, 4897–4902.
Chen, K. C., & Buchanan, T. M. (1980). Hydrolases from Neisseria gonorrhoeae. The study of gonocosin, an aminopeptidase-P, a proline iminopeptidase, and an asparaginase. The Journal of Biological Chemistry, 255, 1704–1710.
Kitazono, A., Kitano, A., Tsuru, D., & Yoshimoto, T. (1994). Isolation and characterization of the prolyl aminopeptidase gene (pap) from Aeromonas sobria: comparison with the Bacillus coagulans enzyme. Journal of Biochemistry, 116, 818–825.
Stepaniak, L. (2000). Isolation and characterization of proline iminopeptidase from Propionibacterium freudenreichii ATCC 9614. Die Nahrung, 44, 102–106.
Leenhouts, K., Bolhuis, A., Boot, J., Deutz, I., Toonen, M., Venema, G., et al. (1998). Cloning, expression, and chromosomal stabilization of the Propionibacterium shermanii proline iminopeptidase gene (pip) for food-grade application in Lactococcus lactis. Applied and Environmental Microbiology, 64, 4736–4742.
Szawlowska, U., Grabowska, A., Zdunek-Zastocka, E., & Bielawski, W. (2012). TsPAP1 encodes a novel plant prolyl aminopeptidase whose expression is induced in response to suboptimal growth conditions. Biochemical and Biophysical Research Communications, 419, 104–109.
Mahon, C. S., O'Donoghue, A. J., Goetz, D. H., Murray, P. G., Craik, C. S., & Tuohy, M. G. (2009). Characterization of a multimeric, eukaryotic prolyl aminopeptidase: an inducible and highly specific intracellular peptidase from the non-pathogenic fungus Talaromyces emersonii. Microbiology (Reading, England), 155, 3673–3682.
Rawlings, N. D., Waller, M., Barrett, A. J., & Bateman, A. (2013). MEROPS: the database of proteolytic enzymes, their substrates and inhibitors. Nucleic Acids Research, 42(D1), D503–D509.
Machida, M. (2002). Progress of Aspergillus oryzae genomics. Advances in Applied Microbiology, 51, 81–106.
Matsushita-Morita, M., Tada, S., Suzuki, S., Hattori, R., Marui, J., Furukawa, I., et al. (2011). Overexpression and characterization of an extracellular leucine aminopeptidase from Aspergillus oryzae. Current Microbiology, 62, 557–564.
Watanabe, J., Tanaka, H., Akagawa, T., Mogi, Y., & Yamazaki, T. (2007). Characterization of Aspergillus oryzae Aspartyl Aminopeptidase Expressed in Escherichia coli. Bioscience, Biotechnology, and Biochemistry, 71, 2557–2560.
Kusumoto, K. I., Matsushita-Morita, M., Furukawa, I., Suzuki, S., Yamagata, Y., Koide, Y., et al. (2008). Efficient production and partial characterization of aspartyl aminopeptidase from Aspergillus oryzae. Journal of Applied Microbiology, 105, 1711–1719.
Matsushita-Morita, M., Furukawa, I., Suzuki, S., Yamagata, Y., Koide, Y., Ishida, H., et al. (2010). Characterization of recombinant prolyl aminopeptidase from Aspergillus oryzae. Journal of Applied Microbiology, 109, 156–165.
Marui, J., Matsushita-Morita, M., Tada, S., Hattori, R., Suzuki, S., Amano, H., et al. (2012). Comparison of expression and enzymatic properties of Aspergillus oryzae lysine aminopeptidases ApsA and ApsB. World Journal of Microbiology and Biotechnology, 28, 2643–2650.
Marui, J., Matsushita-Morita, M., Tada, S., Hattori, R., Suzuki, S., Amano, H., et al. (2012). Enzymatic properties of the glycine d-alanine aminopeptidase of Aspergillus oryzae and its activity profiles in liquid-cultured mycelia and solid-state rice culture (rice koji). Applied Microbiology and Biotechnology, 93, 655–669.
Matsushita-Morita, M., Nakagawa, H., Tada, S., Marui, J., Hattori, R., Suzuki, S., et al. (2013). Characterization of a (D)-stereoselective aminopeptidase (DamA) exhibiting aminolytic activity and halophilicity from Aspergillus oryzae. Applied Biochemistry and Biotechnology, 171, 145–164.
Machida, M., Asai, K., Sano, M., Tanaka, T., Kumagai, T., Terai, G., et al. (2005). Genome sequencing and analysis of Aspergillus oryzae. Nature, 438, 1157–1161.
Atlan, D., Gilbert, C., Blanc, B., & Portalier, R. (1994). Cloning, sequencing and characterization of the pepIP gene encoding a proline iminopeptidase from Lactobacillus delbrueckii subsp. bulgaricus CNRZ 397. Microbiology (Reading, England), 140, 527–535.
Bradford, M. M. (1976). A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Analytical Biochemistry, 72, 248–254.
Yoshimoto, T. (2007). Biochemistry and structural biology of microbial enzymes and their medical applications. Journal of the Pharmaceutical Society of Japan, 127, 1035–1045.
Laemmli, U. K. (1970). Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature, 227, 680–685.
Lobley, A., Sadowski, M. I., & Jones, D. T. (2009). pGenTHREADER and pDomTHREADER: new methods for improved protein fold recognition and superfamily discrimination. Bioinformatics (Oxford, England), 25, 1761–1767.
Basten, D. E., Moers, A. P., Ooyen, A. J., & Schaap, P. J. (2005). Characterisation of Aspergillus niger prolyl aminopeptidase. Molecular Genetics and Genomics : MGG, 272, 673–679.
Mitta, M., Miyagi, M., Kato, I., & Tsunasawa, S. (1998). Identification of the catalytic triad residues of porcine liver acylamino acid-releasing enzyme. Journal of Biochemistry, 123, 924–931.
Sreerama, N., Venyaminov, S. Y., & Woody, R. W. (1999). Estimation of the number of α-helical and β-strand segments in proteins using circular dichroism spectroscopy. Protein Science, 8, 370–380.
Medrano, F., Alonso, J., Garcia, J., Romero, A., Bode, W., & Gomis-Ruth, F. (1998). Structure of proline iminopeptidase from Xanthomonas campestris pv. citri: a prototype for the prolyl oligopeptidase family. The EMBO Journal, 17, 1–9.
Yoshimoto, T., Kabashima, T., Uchikawa, K., Inoue, T., Tanaka, N., Nakamura, K. T., et al. (1999). Crystal structure of prolyl aminopeptidase from Serratia marcescens. Journal of Biochemistry, 126, 559–565.
Uraji, M., Arima, J., Uesugi, Y., Iwabuchi, M., & Hatanaka, T. (2007). Effect of salt on the activity of Streptomyces prolyl aminopeptidase. Biochimica et Biophysica Acta, 1774, 1462–1469.
Hiwatashi, K., Hori, K., Takahashi, K., Kagaya, A., Inoue, S., Sugiyama, T., et al. (2004). Purification and characterization of a novel prolyl aminopeptidase from Maitake (Grifola frondosa). Bioscience, Biotechnology, and Biochemistry, 68, 1395–1397.
Kitazono, A., Kitano, A., Kabashima, T., Ito, K., & Yoshimoto, T. (1996). Prolyl aminopeptidase is also present in Enterobacteriaceae: cloning and sequencing of the Hafnia alvei enzyme-gene and characterization of the expressed enzyme. Journal of Biochemistry, 119, 468–474.
Li, N., Wu, J. M., Zhang, L. F., Zhang, Y. Z., & Feng, H. (2010). Characterization of a unique proline iminopeptidase from white-rot basidiomycetes Phanerochaete chrysosporium. Biochimie, 92, 779–788.
Acknowledgments
This work was supported by the National High Technology Research and Development Program of China (863 Program, 2011AA100905) and the Program for New Century Excellent Talents in University (NCET-12-0878).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ding, GW., Zhou, ND. & Tian, YP. Over-Expression of a Proline Specific Aminopeptidase from Aspergillus oryzae JN-412 and Its Application in Collagen Degradation. Appl Biochem Biotechnol 173, 1765–1777 (2014). https://doi.org/10.1007/s12010-014-0963-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-014-0963-6