Abstract
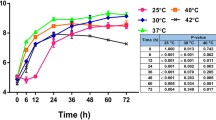
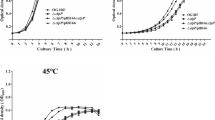
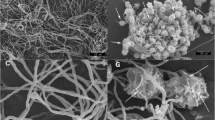
The copper response of Proteus hauseri ZMd44 was determined using one-dimensional (1D) gel electrophoresis coupled with MALDI-TOF-TOF mass spectrometry for a similarity analysis of proteins isolated from P. hauseri ZMd44 cultured in CuSO4-bearing LB medium. Candidate proteins identified as a copper-transporting P-type ATPase (CTPP), phosphoenolpyruvate carboxykinase (PEPCK), flagellin (Fla), and outer membrane proteins (Omps) were the major copper-associated proteins in P. hauseri. In a comparative analysis of subcellular (i.e., periplasmic, intracellular, and inner membranes) and cellular debris, proteomics analysis revealed a distinct differential expression of proteins in P. hauseri with and without copper ion exposure. These findings were consistent with the transcription level dynamics determined using quantitative real-time PCR. Based on a genetic cluster analysis of copper-associated proteins from P. hauseri, Fla and one of the Omps showed greater diversity in their protein sequences compared to those of other Proteus species. Transmission electron microscopy (TEM) and the observed growth on LB agar plates showed that the swarming motility of cells was significantly suppressed and inhibited upon Cu(II) exposure. Thus, copper stress could have important therapeutic significance due to the loss of swarming motility capacity in P. hauseri, which causes urinary tract infections.






Similar content being viewed by others
References
Roberts, S. A., Weichsel, A., Grass, G., Thakali, K., Hazzard, J. T., Tollin, G., Rensing, C., & Montfort, W. R. (2002). Crystal structure and electron transfer kinetics of CueO, a multicopper oxidase required for copper homeostasis in Escherichia coli. Proceedings of the National Academy of Sciences, 99(5), 2766–2771.
Elguindi, J., Hao, X., Lin, Y., Alwathnani, H. A., Wei, G., & Rensing, C. (2011). Advantages and challenges of increased antimicrobial copper use and copper mining. Applied Microbiology and Biotechnology, 91(2), 237–249.
Boal, A. K., & Rosenzweig, A. C. (2009). Structural biology of copper trafficking. Chemical Reviews, 109(10), 4760–4779.
Magnani, D., & Solioz, M. (2007). How bacteria handle copper (pp. 259–285). Berlag: Molecular microbiology of heavy metals. Springer.
Villafane, A. A., Voskoboynik, Y., Cuebas, M., Ruhl, I., & Bini, E. (2009). Response to excess copper in the hyperthermophile Sulfolobus solfataricus strain 98/2. Biochemical and Biophysical Research Communications, 385(1), 67–71.
Rademacher, C., & Masepohl, B. (2012). Copper-responsive gene regulation in bacteria. Microbiology, 158(Pt 10), 2451–2464.
Paulino, L. C., de Mello, M. P., & Ottoboni, L. M. (2002). Differential gene expression in response to copper in Acidithiobacillus ferrooxidans analyzed by RNA arbitrarily primed polymerase chain reaction. Electrophoresis, 23(4), 520–527.
Das, A., Modak, J. M., & Natarajan, K. A. (1998). Surface chemical studies of Thiobacillus ferrooxidans with reference to copper tolerance. Antonie van Leeuwenhoek, 73(3), 215–222.
Rensing, C., & Grass, G. (2003). Escherichia coli mechanism of copper homeostasis in a changing environment. FEMS Microbiology Reviews, 27(2–3), 197–213.
Outten, F. W., Huffman, D. L., Hale, J. A., & O’Halloran, T. V. (2001). The independent cue and cus system confer copper tolerance during aerobic and anaerobic growth in Escherichia coli. Journal of Biological Chemistry, 276(33), 30670–30677.
Franke, S., Grass, G., Rensing, C., & Nies, D. H. (2003). Molecular analysis of the copper-transporting efflux system CusCFBA of Escherichia coli. Journal of Bacteriology, 185(13), 3804–3812.
Piccini, C. D., & Legnani-Fajardo, C. L. (1998). Identification of iron-regulated outer membrane proteins in uropathogenic Proteus mirabilis and its relationship with heme uptake. FEMS Microbiology Letters, 166(2), 243–248.
Rensing, C., Mitra, B., & Rosen, B. P. (1998). A Zn(II)-translocating P-type ATPase from Proteus mirabilis. Biochemistry and Cell Biology, 76(5), 787–790.
Ojo, A. O., van Heerden, E., & Piater, L. A. (2008). Identification and initial characterization of a copper resistant South African mine isolate. African Journal of Microbiology Research, 2(11), 281–287.
Zheng, X. S., Ng, I. S., Ye, C. M., Chen, B. Y., & Lu, Y. H. (2013). Copper ion-stimulated McoA-laccase production and enzyme characterization in Proteus hauseri ZMd44. Journal of Bioscience and Bioengineering, 115(4), 388–393.
Zhang, M. M., Chen, W. M., Chen, B. Y., Chang, C. T., Hsueh, C. C., Ding, Y. T., Lin, K. L., & Xu, H. Z. (2010). Comparative study on characteristics of azo dye decolorization by indigenous decolorizers. Bioresource Technology, 101(8), 2651–2656.
Chen, B. Y., Zhang, M. M., Chang, C. T., Ding, Y. T., Lin, K. L., Chiou, C. S., Hsueh, C. C., & Xu, H. Z. (2010). Assessment upon azo dye decolorization and bioelectricity generation by Proteus hauseri. Bioresource Technology, 101(12), 4737–4741.
Grass, G., & Rensing, C. (2001). CueO is a multi-copper oxidase that confers copper tolerance in Escherichia coli. Biochemical and Biophysical Research Communications, 286(5), 902–908.
Galhaup, C., & Haltrich, D. (2001). Enhanced formation of laccase activity by the white-rot fungus Trametes pubescens in the presence of copper. Applied Microbiology and Biotechnology, 56(1–2), 225–232.
Ng, I. S., Xu, F., Ye, C., Chen, B. Y., & Lu, Y. (2014). Exploring metal effects and synergistic interactions of ferric stimulation on azo-dye decolorization by new indigenous Acinetobacter guillouiae Ax-9 and Rahnella aquatilis DX2b. Bioprocess and Biosystems Engineering, 37(2), 217–224. doi:10.1007/s00449-013-0988-1.
Kanamaru, K., Kashiwaqi, S., & Mizuno, T. (1994). A copper-transporting P-type ATPase found in the thylakoid membrane of the cyanobacterium Synechococcus species PCC7942. Molecular Microbiology, 13(2), 369–377.
Barry, A. N., Shinde, U., & Lutsenko, S. (2010). Structural organization of human Cu-transporting ATPases: learning from building blocks. Journal of Biological Inorganic Chemistry, 15(1), 47–59.
Delbaer, L. T., Sudom, A. M., Prasad, L., Leduc, Y., & Goldie, H. (2004). Structure/function studies of phosphoryl transfer by phosphoenolpyruvate carboxykinase. Biochimica et Biophysica Acta (BBA) - Proteins and Proteomics, 1697(1–2), 271–278.
Ng, I. S., Zheng, X. S., Chen, B. Y., Chi, X. Q., Lu, Y. H., & Chang, C. S. (2013). Proteomics approach to decipher novel genes and enzymes characterization of a bioelectricity-generating and dye-decolorizing bacterium Proteus hauseri ZMd44. Biotechnology and Bioprocess Engineering, 18(1), 8–17.
Navarro, C. A., Orellana, L. H., Mauriaca, C., & Jerez, C. A. (2009). Transcriptional and functional studies of Acidithiobacillus ferrooxidans genes related to survival in the presence of copper. Applied and Environmental Microbiology, 75(19), 6102–6109.
Teitzel, G. M., Geddie, A., Susan, K., Kirisits, M. J., Whiteley, M., & Parsek, M. R. (2006). Survival and growth in the presence of elevated copper: transcriptional profiling of copper-stressed Pseudomonas aeruginosa. Journal of Bacteriology, 188(20), 7242–7256.
Jones, B. V., Young, R., Mahenthiralingam, E., & Stickler, D. J. (2004). Ultrastructure of Proteus mirabilis swarmer cell rafts and role of swarming in catheter-associated urinary tract infection. Infection and Immunity, 72(7), 3941–3950.
Armbruster, C. E., & Mobley, H. L. (2012). Merging mythology and morphology: the multifaceted lifestyle of Proteus mirabilis. Nature Reviews Microbiology, 10, 743–754.
Tuson, H. H., Copeland, M. F., Carey, S., Sacotte, R., & Weibel, D. B. (2013). Flagellum density regulates Proteus mirabilis swarmer cell motility in viscous environments. Journal of Bacteriology, 195(2), 368–377.
Olukanni, O. D., Osuntoki, A. A., Kalyani, D. C., Gbenle, G. O., & Govindwar, S. P. (2010). Decolorization and biodegradation of Reactive Blue 13 by Proteus mirabilis LAG. Journal of Hazardous Materials, 184(1), 290–298.
Chen, B. Y., Wang, Y. M., & Ng, I. S. (2011). Understanding interactive characteristics of bioelectricity generation and reductive decolorization using Proteus hauseri. Bioresource Technology, 102(2), 1159–1165.
Pearson, M. M., Sebaihia, M., Churcher, C., Quail, M. A., Seshasayee, A. S., Luscombe, N. M., Abdellah, Z., Arrosmith, C., Atkin, B., & Chillingworth, T. (2008). Complete genome sequence of uropathogenic Proteus mirabilis, a master of both adherence and motility. Journal of Bacteriology, 190(11), 4027–4037.
Nielubowicz, G. R., Smith, S. N., & Mobley, H. L. (2010). Zinc uptake contributes to motility and provides a competitive advantage to Proteus mirabilis during experimental urinary tract infection. Infection and Immunity, 78(6), 2823–2833.
Mobley, H. L., Belas, R., Lockatell, V., Chippendale, G., Trifillis, A. L., Johnson, D. E., & Warren, J. W. (1996). Construction of a flagellum-negative mutant of Proteus mirabilis: effect on internalization by human renal epithelial cells and virulence in a mouse model of ascending urinary tract infection. Infection and Immunity, 64(12), 5332–5340.
Hermes-Lima, M., & Vieyra, A. (1992). Pyrophosphate synthesis from phospho(enol)pyruvate catalyzed by precipitated magnesium phosphate with “enzyme-like” activity. Journal of Molecular Evolution, 35(4), 277–285.
de Zwart, I. I., Meade, S. J., & Pratt, A. J. (2004). Biomimetic phosphoryl transfer catalysed by iron(II)-mineral precipitates. Geochimica et Cosmochimica Acta, 68(20), 4093–4098.
Kearns, D. B. (2010). A field guide to bacterial swarming motility. Nature Reviews Microbiology, 8(9), 634–644.
Acknowledgments
The authors are grateful to the financial support by the Fundamental Research Funds for the Central Universities (2011121017), the National Natural Science Foundation of China (21206141), and the Fujian Provincial Department of Science & Technology (2012I0009). The authors also sincerely appreciate the academic connection program between Xiamen University (China) and National I-Lan University (Taiwan) in 2011–2014 for the study.
Author information
Authors and Affiliations
Corresponding author
Additional information
Xuesong Zheng and Nan Wang contributed equally to this work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Fig. S1
(DOC 399 kb)
Rights and permissions
About this article
Cite this article
Ng, IS., Zheng, X., Wang, N. et al. Copper Response of Proteus hauseri Based on Proteomic and Genetic Expression and Cell Morphology Analyses. Appl Biochem Biotechnol 173, 1057–1072 (2014). https://doi.org/10.1007/s12010-014-0892-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-014-0892-4